The researchers have revealed the exact molecular structure of an IgM-type B cell receptor.
Freiburg and Harvard University researchers have revealed the three-dimensional structure of the B cell antigen receptor, providing new insight into its composition.
B cells have antigen receptors on their surface that allow them to identify invading pathogens such as bacteria and viruses. When a B cell receptor binds to an antigen, that is, to a foreign structure, the B cell is activated which triggers the production of antibodies. Antibodies are critical for human survival because they protect us from severe diseases from infections with pathogens such as COVID-19. Vaccines provide protection by activating antigen receptors, triggering an immune response.
An international collaboration of researchers from the University of Freiburg’s Cluster of Excellence CIBSS and Harvard Medical School in the United States has recently revealed the exact molecular structure of an IgM-type B cell receptor. Their results suggest that the B cell’s surface receptor interacts with other receptors, thus controlling signal transduction. The findings were recently published in the prestigious journal Nature.
Connection of Signaling Subunits with the Immunoglobulin
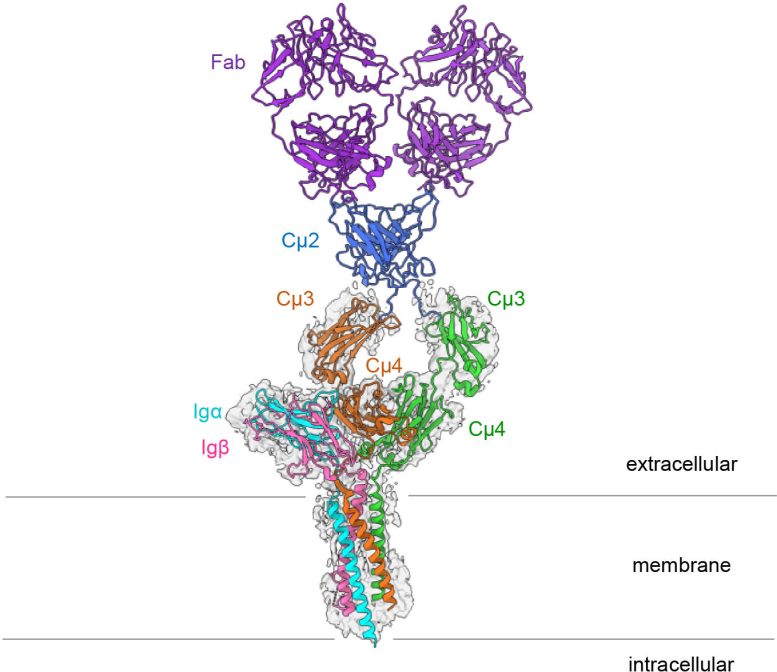
The B cell antigen receptor is made up of an antibody linked to the cell membrane as well as two smaller proteins known as Ig alpha and Ig beta. When the B cell receptor detects a pathogen, these smaller subunits transmit signals to the cell’s interior.
“Exactly how these signaling subunits are connected with the immunoglobulin was previously unknown,” says Prof. Dr. Michael Reth from the University of Freiburg’s Faculty of Biology, who has been conducting research on the receptor for over 30 years and originally discovered its signaling subunits. He is a member of the Cluster of Excellence CIBSS – Centre for Integrative Biological Signalling Studies and co-director of the Cluster of Excellence BIOSS.
“For a long time, we did not have the technical possibilities to study the exact structure of membrane proteins. Now, cryo-electron microscopy has enabled us to create a high-resolution image of the B cell receptor,” says Reth.
With cryo-electron microscopy, the sample to be studied is cooled very rapidly to minus 183 °C. This reduces the natural movement of the molecules and prevents the formation of tiny ice crystals that otherwise would destroy the protein structure. In this way, it is possible to achieve resolutions that are many times higher than with other electron microscopic methods. In their current study, the researchers achieved a resolution of 3.3 ångströms, which corresponds to the width of just a few atoms. To do so, they combined hundreds of thousands of images of the entire receptor with those of a truncated version that lacked two flexible regions. They then used these data to calculate the complete three-dimensional structure of the B cell receptor on the computer.
A symmetrical membrane-bound antibody binds only on one side
The striking thing about the three-dimensional structure is that the symmetrical membrane-bound antibody only binds to Ig alpha and Ig beta on one side, thus forming an asymmetrical complex. This asymmetry resembles that of the T cell receptor, another important immune receptor whose structure was first elucidated in 2019. “It is astounding that both types of antigen receptor form asymmetrical complexes,” explains Reth. “This leads us to conclude that the structure now elucidated is part of a larger receptor complex and that it interacts with still other molecules on the B cell surface.”
Such larger structures, which are held together through less powerful forces, cannot yet be studied with techniques like cryo-electron microscopy. However, the newly published molecular structure provides further evidence in favor of such an interaction with other molecules: It shows that the outside of the B cell receptor contains conserved amino acids. Amino acids are described as conserved if they hardly change in the course of evolution and are therefore identical in the antigen receptors of different organisms. “The presence of conserved amino acids that are directed outward suggests that the IgM B cell receptor has further binding partners,” says Reth. “In other words, we only know part of the machine so far – and now we want to identify the other building blocks and determine how they influence the signaling effect of the receptor.”
These other building blocks could explain how the receptor is normally kept quiescent and is activated only when it binds to an antigen. “That will be one of the next important tasks in the study of adaptive immunity,” summarizes Reth. “A better understanding of B cell activation could also help us to further improve the development of vaccines or to understand the formation of lymphoma in which the B cell receptor is activated in an uncontrolled manner.”
Reference: “Structural principles of B cell antigen receptor assembly” by Ying Dong, Xiong Pi, Frauke Bartels-Burgahn, Deniz Saltukoglu, Zhuoyi Liang, Jianying Yang, Frederick W. Alt, Michael Reth and Hao Wu, 13 October 2022, Nature.
DOI: 10.1038/s41586-022-05412-7
The study was funded by the National Institutes of Health (NIH) and the German Research Foundation (DFG).






Be the first to comment on "Scientists Unravel the Molecular Structure of One of the Most Important Immune Receptors"