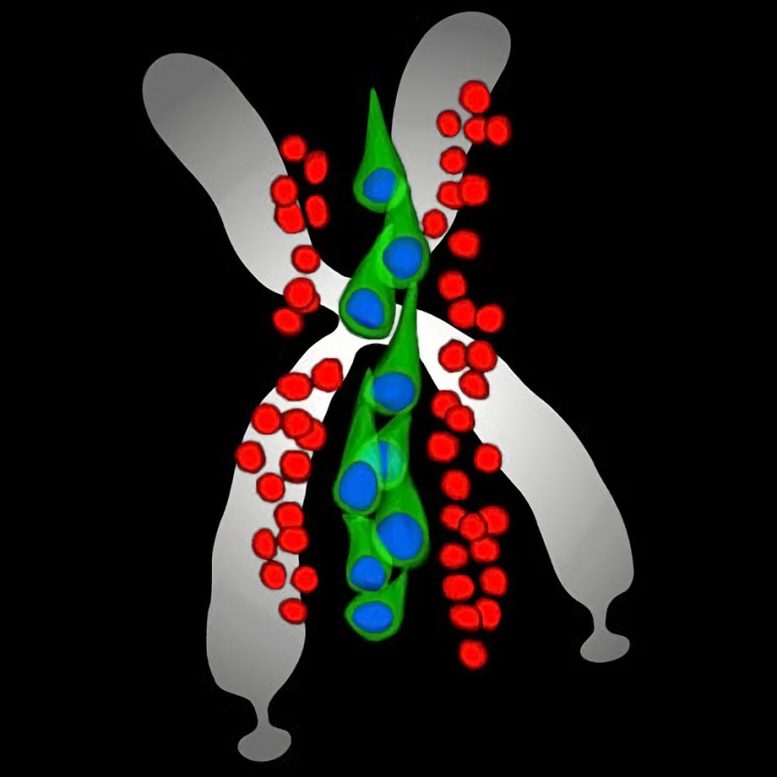
Artist representation of early developmental brain cells that when disrupted cause Fragile X syndrome. Credit: Yale University
A new study from neurobiologists at Yale University examines the evolutionary changes surrounding the NOS1 gene and suggests that the same evolutionary mechanisms that may have gifted our species with amazing cognitive abilities may have also made us more susceptible to disorders such as autism.
A recently evolved pattern of gene activity in the language and decision-making centers of the human brain is missing in a disorder associated with autism and learning disabilities, a new study by Yale University researchers shows.
“This is the cost of being human,” said Nenad Sestan, associate professor of neurobiology, researcher at Yale’s Kavli Institute for Neuroscience, and senior author of the paper. “The same evolutionary mechanisms that may have gifted our species with amazing cognitive abilities have also made us more susceptible to psychiatric disorders such as autism.”
The findings are reported in the May 11 issue of the journal Cell.
In the Cell paper, Kenneth Kwan, the lead author, and other members of the Sestan laboratory identified the evolutionary changes that led the NOS1 gene to become active specifically in the parts of the developing human brain that form the adult centers for speech and language and decision-making. This pattern of NOS1 activity is controlled by a protein called FMRP and is missing in Fragile X syndrome, a disorder caused by a genetic defect on the X chromosome that disrupts FMRP production. Fragile X syndrome, the leading inherited form of intellectual disability, is also the most common single-gene cause of autism. The loss of NOS1 activity may contribute to some of the many cognitive deficits suffered by those with Fragile X syndrome, such as lower IQ, attention deficits, and speech and language delays, the authors say.
The pattern of NOS1 activity in these brain centers does not occur in the developing mouse brain — suggesting that it is a more recent evolutionary adaptation possibly involved in the wiring of neural circuits important for higher cognitive abilities. The findings of the Cell paper support this hypothesis. The study also provides insights into how genetic deficits in early development, a time when brain circuits are formed, can lead to disorders such as autism, in which symptoms appear after birth.
“This is an example of where the function of genetic changes that likely drove aspects of human brain evolution was disrupted in disease, possibly reverting some of our newly acquired cognitive abilities and thus contributing to a psychiatric outcome,” Kwan said.
Reference: “Species-Dependent Posttranscriptional Regulation of NOS1 by FMRP in the Developing Cerebral Cortex” by Kenneth Y. Kwan, Mandy M.S. Lam, Matthew B. Johnson, Umber Dube, Sungbo Shim, Mladen-Roko Rašin, André M.M. Sousa, Sofia Fertuzinhos, Jie-Guang Chen, Jon I. Arellano, Daniel W. Chan, Mihovil Pletikos, Lana Vasung, David H. Rowitch, Eric J. Huang, Michael L. Schwartz, Rob Willemsen, Ben A. Oostra, Pasko Rakic, Marija Heffer, Ivica Kostovic, Milos Judaš and Nenad Šestan, 11 May 2012, Cell.
DOI: 10.1016/j.cell.2012.02.060
More than 20 U.S. and international scientists contributed to the research, which was supported by grants from the National Institutes of Health and the Kavli Foundation.







Be the first to comment on "Evolutionary Changes Surrounding the NOS1 Gene"