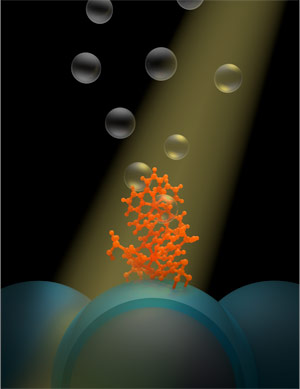
Tom Meyer at the Energy Frontier Research Center at the University of North Carolina at Chapel Hill built a device that converts the sun’s energy not into electricity but hydrogen fuel and stores it for later use. The device, a dye-sensitized photoelectrosynthesis cell generates hydrogen fuel by using the sun’s energy to split water into its component parts. After the split, hydrogen is sequestered and stored, while the byproduct, oxygen, is released into the air. Credit: Yan Liang
A new system designed by researchers at UNC and NC State converts the sun’s energy into hydrogen fuel and stores it for later use.
Solar energy has long been used as a clean alternative to fossil fuels such as coal and oil, but it could only be harnessed during the day when the sun’s rays were strongest. Now researchers led by Tom Meyer at the Energy Frontier Research Center at the University of North Carolina at Chapel Hill have built a system that converts the sun’s energy not into electricity but hydrogen fuel and stores it for later use, allowing us to power our devices long after the sun goes down.
“So called ‘solar fuels’ like hydrogen offer a solution to how to store energy for nighttime use by taking a cue from natural photosynthesis,” said Meyer, Arey Distinguished Professor of Chemistry at UNC’s College of Arts and Sciences. “Our new findings may provide a last major piece of a puzzle for a new way to store the sun’s energy – it could be a tipping point for a solar energy future.”
In one hour, the sun puts out enough energy to power every vehicle, factory, and device on the planet for an entire year. Solar panels can harness that energy to generate electricity during the day. But the problem with the sun is that it goes down at night—and with it the ability to power our homes and cars. If solar energy is going to have a shot at being a clean source for powering the planet, scientists had to figure out how to store it for night-time use.
The new system designed by Meyer and colleagues at UNC and with Greg Parsons’ group at North Carolina State University does exactly that. It is known as a dye-sensitized photoelectrosynthesis cell, or DSPEC, and it generates hydrogen fuel by using the sun’s energy to split water into its component parts. After the split, hydrogen is sequestered and stored, while the byproduct, oxygen, is released into the air.
“But splitting water is extremely difficult to do,” said Meyer. “You need to take four electrons away from two water molecules, transfer them somewhere else, and make hydrogen, and, once you have done that, keep the hydrogen and oxygen separated. How to design molecules capable of doing that is a really big challenge that we’ve begun to overcome.”
Meyer had been investigating DSPECs for years at the Energy Frontier Research Center at UNC and before. His design has two basic components: a molecule and a nanoparticle. The molecule, called a chromophore-catalyst assembly, absorbs sunlight and then kick starts the catalyst to rip electrons away from water. The nanoparticle, to which thousands of chromophore-catalyst assemblies are tethered, is part of a film of nanoparticles that shuttles the electrons away to make the hydrogen fuel.
However, even with the best of attempts, the system always crashed because either the chromophore-catalyst assembly kept breaking away from the nanoparticles or because the electrons couldn’t be shuttled away quickly enough to make hydrogen.
To solve both of these problems, Meyer turned to the Parsons group to use a technique that coated the nanoparticle, atom by atom, with a thin layer of a material called titanium dioxide. By using ultra-thin layers, the researchers found that the nanoparticle could carry away electrons far more rapidly than before, with the freed electrons available to make hydrogen. They also figured out how to build a protective coating that keeps the chromophore-catalyst assembly tethered firmly to the nanoparticle, ensuring that the assembly stays on the surface.
With electrons flowing freely through the nanoparticle and the tether stabilized, Meyer’s new system can turn the sun’s energy into fuel while needing almost no external power to operate and releasing no greenhouse gases. What’s more, the infrastructure to install these sunlight-to-fuel converters is in sight based on existing technology. A next target is to use the same approach to reduce carbon dioxide, a greenhouse gas, to a carbon-based fuel such as formate or methanol.
“When you talk about powering a planet with energy stored in batteries, it’s just not practical,” said Meyer. “It turns out that the most energy-dense way to store energy is in the chemical bonds of molecules. And that’s what we did – we found an answer through chemistry.”
References:
“Solar water splitting in a molecular photoelectrochemical cell” by Leila Alibabaei, M. Kyle Brennaman, Michael R. Norris, Berç Kalanyan, Wenjing Song, Mark D. Losego, Javier J. Concepcion, Robert A. Binstead, Gregory N. Parsons and Thomas J. Meyer, 25 November 2013, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.1319628110
“A Sensitized Nb2O5 Photoanode for Hydrogen Production in a Dye-Sensitized Photoelectrosynthesis Cell” by Hanlin Luo, Wenjing Song, Paul G. Hoertz, Kenneth Hanson, Rudresh Ghosh, Sylvie Rangan, M. Kyle Brennaman, Javier J. Concepcion, Robert A. Binstead, Robert Allen Bartynski, Rene Lopez and Thomas J. Meyer, 28 December 2012, Chemistry Materials.
DOI: 10.1021/cm3027972








I’m disappointed that this article didn’t even attempt to discuss the energy conversion (quantum) efficiency if the process. Certainly the achievement described is very impressive. But leaving out the energy conversion rates makes the article weak at best
I agree wholeheartedly. What about the cost of the titanium dioxide coating? What about the energy needed to compress into liquid hydrogen–if stored that way? What about the use of fuel cell?
I also agree with the statement regarding energy storage in chemical bonds rather than electric charge in a battery being the answer. Like the bonds that are found in the hydrocarbons of oil for example?
Likewise, the sun puts out enough energy in an hour but over what area? 4pi steradians, the earths area, earths land area?
These articles must have a few real facts and figures even if in a “fact box” to capture my interest. Perhaps the writer does not have the skills to ask the questions or the editor leaves out the boring bit for the mythical general reader.
Anthony Lealand
Isn’t oxygen volatile? How do you solve the problem of releasing it without catastrophic explosions?
Its not pure oxygen or an oxygenizer like n02 and if it was you can simply release it through water bubblers………..
What happens to the water? Is it consumed for ever or does H2O combine at a latter time? Seems to me water would become a fossil fuel.
Water is water and will be more purified than it went in when it comes out. Hydrogen cars have a water trap and when it gets full it expells the water right onto the ground…………..California will have an answer to solving their droughts after their hydrogen cars start surpassing 100k vehicles as hydrogen cars emit just as much water as a gas vehicle consumes fuel…….Right now theres under 20k on the road currently with only 45 filling stations..
I don’t think I’m ready to attach this unit or mechanism to my solar panels – maybe in ten years, when a lot of the problems are worked out.