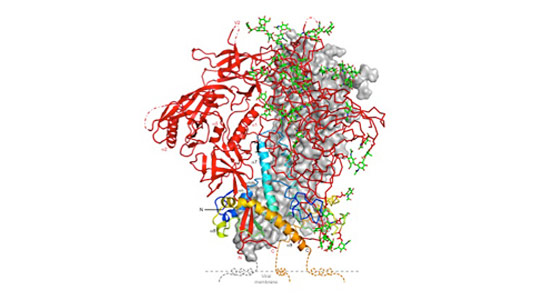
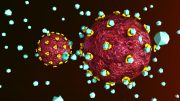
Structure of the HIV spike protein in its closed state, which makes it less detectable to immune system. Credit: Peter Kwong
In two newly published studies, researchers detail the structure and dynamics of the HIV spike protein, which is used by the virus uses to fuse with and enter cells.
HIV is adept at eluding immune system responses because the protein it uses to infect cells is constantly changing.
Now a team of researchers including scientists from Yale have stripped the cloak from this master of disguise, providing a high-resolution image of this surface spike protein and monitoring how it constantly changes its shape, information that suggests new ways to attack the virus through drugs and vaccines.
In two papers published simultaneously online on October 8 in the journals Science and Nature, a team of researchers led by scientists from the labs of Walther Mothes at Yale University, Peter Kwong at Vaccine Research Center at the National Institute of Allergy and Infectious Diseases and Scott Blanchard at Weill Cornell Medical College describe the structure and dynamics of the HIV spike protein, which the virus uses to fuse with and enter cells.
“Now we can see how this fusion machine works, and in a general way it is similar to how fusion works in influenza and Ebola,” said Mothes, associate professor of microbial pathogenesis and co-senior author of the Science paper.
The spike protein needs to be in “an open state” to fuse with and infect cells. However, in its closed state, it is less visible to antibodies. Thus, the spike protein tries to stay longer in the closed state and only briefly opens, making it more difficult to attack the virus.
Mothes noted that new research explains why a class of antibodies – discovered in few AIDS patients – offer protection against the disease. These broadly neutralizing antibodies keep this spike protein closed and thereby prevent the spread of the virus.
“The determination of the structure of this closed configuration of the HIV spike protein and the direct visualization of its fast openings represent a major step forward for drug and vaccine design,” Mothes said.
James B. Munro of Yale is the lead author of the Science paper. Blanchard of Cornell is co-senior author. Kwong of the NIAID is senior author of the Nature paper.
Funding for the studies were provided by the National Institutes of Health, The Cancer Research Institute, the China Scholarships Council, the International AIDS Vaccine Initiative, the Bill & Melinda Gates Foundation, and the United States Agency for International Development (USAID).
References:
“Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions” by James B. Munro, Jason Gorman, Xiaochu Ma, Zhou Zhou, James Arthos, Dennis R. Burton, Wayne C. Koff, Joel R. Courter, Amos B. Smith, Iii, Peter D. Kwong, Scott C. Blanchard and Walther Mothes, 8 October 2014, Science.
DOI: 10.1126/science.1254426
“Structure and immune recognition of trimeric pre-fusion HIV-1 Env” by Marie Pancera, Tongqing Zhou, Aliaksandr Druz, Ivelin S. Georgiev, Cinque Soto, Jason Gorman, Jinghe Huang, Priyamvada Acharya, Gwo-Yu Chuang, Gilad Ofek, Guillaume B. E. Stewart-Jones, Jonathan Stuckey, Robert T. Bailer, M. Gordon Joyce, Mark K. Louder, Nancy Tumba, Yongping Yang, Baoshan Zhang, Myron S. Cohen, Barton F. Haynes, John R. Mascola, Lynn Morris, James B. Munro, Scott C. Blanchard, Walther Mothes, Mark Connors and Peter D. Kwong, 8 October 2014, Nature.
DOI:10.1038/nature13808




Be the first to comment on "Researchers Detail the Structure and Dynamics of the HIV Spike Protein"