Atom Illustration
What Are Atomic Nuclei?
In 1911, Ernest Rutherford discovered that at the core of every atom is a nucleus. Atomic nuclei consist of electrically positive protons and electrically neutral neutrons. These are held together by the strongest known fundamental force, called the strong force. The nucleus makes up much less than .01% of the volume of the atom, but typically contains more than 99.9% of the mass of the atom.
The chemical properties of a substance are determined by the negatively charged electrons enshrouding the nucleus. The number of electrons usually matches the number of protons in the nucleus. Some nuclei are unstable and may undergo radioactive decay, eventually arriving at a stable state through the emission of photons (gamma decay), emission or capture of electrons or positrons (beta decay), emission of helium nuclei (alpha decay), or a combination of these processes. Most nuclei are spherical or ellipsoidal, though some exotic shapes exist. Nuclei can vibrate and rotate when struck by other particles. Some are unstable and will break apart or change their relative number of protons and neutrons.
Nuclei Facts
- A typical grain of sand contains more than 10 million trillion nuclei. That’s 100 times more than the number of seconds since the beginning of the Universe.
- The nucleus accounts for more than 99.9994% of the total atomic mass, but occupies less than one ten-trillionth of the atomic volume.
- All nuclei have approximately the same density. If the Moon was smashed to the same density, it would fit inside Yankee Stadium.
DOE Office of Science: Contributions to Nuclei Research
The DOE Office of Nuclear Physics in the Office of Science supports research to understand all forms of nuclear matter. This research includes mechanisms that form heavy nuclei in cosmic neutron star mergers. It also includes unraveling previously unknown properties of nuclei in their natural state for important applications in medicine, commerce, and national defense. Another area of study is understanding precisely how nuclei are structured depending on the number of protons and neutrons inside them. Other research focuses on heating nuclei to the temperature of the early universe to understand how they condensed out of the quark-gluon soup that existed at the time.

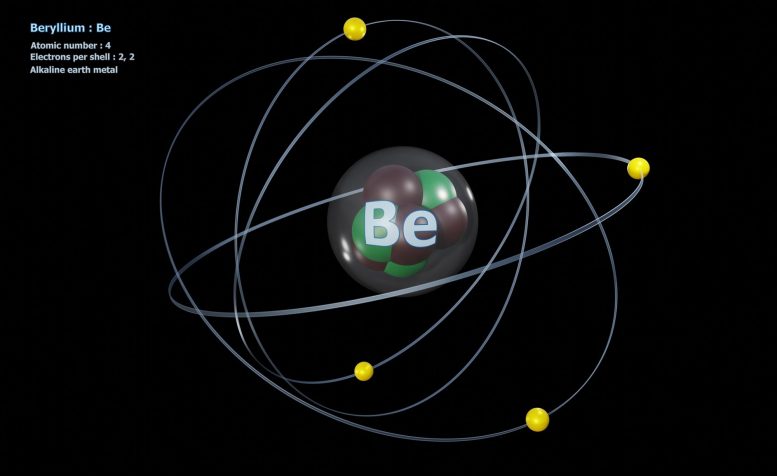





This is the usual model of the atom any real scientist teaches the student. There are some difficulties because it is hard to explain the 4 forces and the large no of quantum particles and why fission always splits atoms into certain fission pairs.
But there are alt science people all over youtube producing high quality graphics videos who are not real physicists teaching various preposterous models that comes out of the “electric universe” ideology. I know several people who believe in it. One is an actual high school physics teacher that tought one of my children basic physics who also teaches on the web that the sun does not do fusion in its core. Stellar fusion requires that neutrons be produced to make helium from plain old BB hydrogen (protons) but they don’t believe in neutrons.
If you see or hear about structured atoms or the electric universe, run a mile. They have seminars that likely have high fees for simpletons. In this model there are no neutrons because every “neutron” is now a proton electron pair where the electron is bound to the proton by it’s charge, and heavier atoms of all sizes are just collections of protons glued together by a smaller no of electrons. Take the carbon atom which should have 6p+6n plus 6e in the orbitals. In the alt model the 6n are now 6p with 6e inside the nucleus ie inner electrons, with electro static charges doing the job of gluons because those don’t exist either. How 6e can bind 12p without busting apart is beyond me. And the very same folks tell us that while neutrons don’t exist, they still decay in 15mins or so.
It gets better, all the elements now have complex shapes alot like complex molecules since the nuclius is really fractional atoms joined hierarchically like molecules in chemistry pics. So uranium ii really formed by joining strontium atom to cesium atom and some extra bits. Thats why they believe fission falls that way. So they conclude the atom is a hierarchy of smaller atoms. Gotta see it to believe it.
Stay away from these alt science folks, they are as bad as flat earthers and it wastes alot of time trying to bring people back to normal science, but we also have to be aware of their existence. They are the Qanon of science. Another group of sciencey idiots are the free energy crowd, they just want their hydrinos and cold fusion to exist so they can have free energy without having to do real engineering. Many are into fraud raising money from investers that don’t understand normal science.