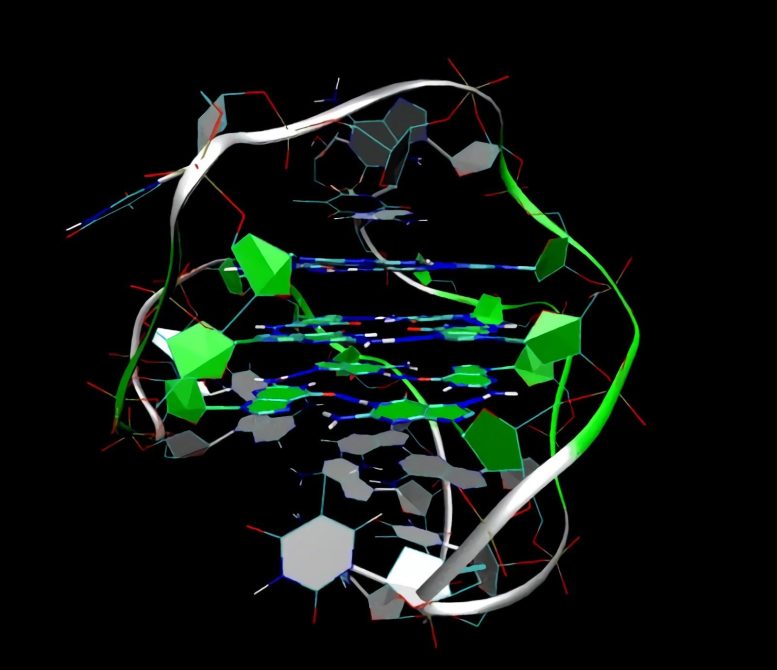
The mapping of telomerase may boost our knowledge of cancers and their treatment, says Stig E. Bojesen. Credit: University of Copenhagen
For the first time, researchers have successfully mapped telomerase, finding that differences in the telomeric gene are associated both with the risk of various cancers and with the length of the telomeres.
In collaboration with an international research team, University of Copenhagen researchers have for the first time mapped telomerase, an enzyme that has a kind of rejuvenating effect on normal cell aging. The findings have just been published in Nature Genetics and are a step forward in the fight against cancer.
Mapping the cellular fountain of youth – telomerase. This is one of the results of a major research project involving more than 1,000 researchers worldwide, four years of hard work, DKK 55 million from the EU and blood samples from more than 200,000 people. This is the largest collaboration project ever to be conducted within cancer genetics.
Stig E. Bojesen, a researcher at the Faculty of Health and Medical Sciences, University of Copenhagen, and staff specialist at the Department of Clinical Biochemistry, Copenhagen University Hospital, Herlev, has headed the efforts to map telomerase – an enzyme capable of creating new ends on cellular chromosomes, the so-called telomeres. In other words, a kind of cellular fountain of youth.
“We have discovered that differences in the telomeric gene are associated both with the risk of various cancers and with the length of the telomeres. The surprising finding was that the variants that caused the diseases were not the same as the ones which changed the length of the telomeres. This suggests that telomerase plays a far more complex role than previously assumed,” says Stig E. Bojesen.
The mapping of telomerase is an important discovery, because telomerase is one of the very basic enzymes in cell biology. It relengthens the telomeres so that they get the same length as before embarking on cell division.
“The mapping of telomerase may, among other things, boost our knowledge of cancers and their treatment, and with the new findings the genetic correlation between cancer and telomere length has been thoroughly illustrated for the first time,” says Stig E. Bojesen.
Telomeres a cellular ‘multi-ride ticket’
The human body consists of 50,000,000,000,000 or fifty trillion cells, and each cell has 46 chromosomes which are the structures in the nucleus containing our hereditary material, the DNA. The ends of all chromosomes are protected by so-called telomeres. The telomeres serve to protect the chromosomes in much the same way as the plastic sheath on the end of a shoelace. But each time a cell divides, the telomeres become a little bit shorter and eventually end up being too short to protect the chromosomes.
Popularly speaking, each cell has a multi-ride ticket, and each time the cell divides, the telomeres (the chromosome ends) will use up one ride. Once there are no more rides left, the cell will not divide any more, and will, so to speak, retire. But some special cells in the body can activate telomerase, which again can elongate the telomeres.
Sex cells, or other stem cells which must be able to divide more than normal cells, have this feature. Unfortunately, cancer cells have discovered the trick, and it is known that they also produce telomerase and thus keep themselves artificially young. The telomerase gene therefore plays an important role in cancer biology, and it is precisely by identifying cancer genes that the researchers imagine that you can improve the identification rate and the treatment.
“A gene is like a country. As you map it, you can see what is going on in the various cities. One of the cities in what could be called Telomerase Land determines whether you develop breast cancer or ovarian cancer, while other parts of the gene determine the length of the telomeres. Mapping telomerase is therefore an important step towards being able to predict the risk of developing different cancers. In summary, our findings are very surprising and point in many directions. But as is the case with all good research, our work provides many answers but leaves even more questions,” says Stig E. Bojesen.
Reference: “Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer” by Stig E Bojesen, Karen A Pooley, Sharon E Johnatty, Jonathan Beesley, Kyriaki Michailidou, Jonathan P Tyrer, Stacey L Edwards, Hilda A Pickett, Howard C Shen, Chanel E Smart, Kristine M Hillman, Phuong L Mai, Kate Lawrenson, Michael D Stutz, Yi Lu, Rod Karevan, Nicholas Woods, Rebecca L Johnston, Juliet D French, Xiaoqing Chen, Maren Weischer, Sune F Nielsen, Melanie J Maranian, Maya Ghoussaini, Shahana Ahmed, Caroline Baynes, Manjeet K Bolla, Qin Wang, Joe Dennis, Lesley McGuffog, Daniel Barrowdale, Andrew Lee, Sue Healey, Michael Lush, Daniel C Tessier, Daniel Vincent, Françis Bacot, Australian Cancer Study, Australian Ovarian Cancer Study, Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab), Gene Environment Interaction and Breast Cancer (GENICA), Swedish Breast Cancer Study (SWE-BRCA), The Hereditary Breast and Ovarian Cancer Research Group Netherlands (HEBON), Epidemiological study of BRCA1 & BRCA2 Mutation Carriers (EMBRACE), Genetic Modifiers of Cancer Risk in BRCA1/2 Mutation Carriers (GEMO), Ignace Vergote, Sandrina Lambrechts, Evelyn Despierre, Harvey A Risch, Anna González-Neira, Mary Anne Rossing, Guillermo Pita, Jennifer A Doherty, Nuria Álvarez, Melissa C Larson, Brooke L Fridley, Nils Schoof, Jenny Chang-Claude, Mine S Cicek, Julian Peto, Kimberly R Kalli, Annegien Broeks, Sebastian M Armasu, Marjanka K Schmidt, Linde M Braaf, Boris Winterhoff, Heli Nevanlinna, Gottfried E Konecny, Diether Lambrechts, Lisa Rogmann, Pascal Guénel, Attila Teoman, Roger L Milne, Joaquin J Garcia, Angela Cox, Vijayalakshmi Shridhar, Barbara Burwinkel, Frederik Marme, Rebecca Hein, Elinor J Sawyer, Christopher A Haiman, Shan Wang-Gohrke, Irene L Andrulis, Kirsten B Moysich, John L Hopper, Kunle Odunsi, Annika Lindblom, Graham G Giles, Hermann Brenner, Jacques Simard, Galina Lurie, Peter A Fasching, Michael E Carney, Paolo Radice, Lynne R Wilkens, Anthony Swerdlow, Marc T Goodman, Hiltrud Brauch, Montserrat Garcia-Closas, Peter Hillemanns, Robert Winqvist, Matthias Dürst, Peter Devilee, Ingo Runnebaum, Anna Jakubowska, Jan Lubinski, Arto Mannermaa, Ralf Butzow, Natalia V Bogdanova, Thilo Dörk, Liisa M Pelttari, Wei Zheng, Arto Leminen, Hoda Anton-Culver, Clareann H Bunker, Vessela Kristensen, Roberta B Ness, Kenneth Muir, Robert Edwards, Alfons Meindl, Florian Heitz, Keitaro Matsuo, Andreas du Bois, Anna H Wu, Philipp Harter, Soo-Hwang Teo, Ira Schwaab, Xiao-Ou Shu, William Blot, Satoyo Hosono, Daehee Kang, Toru Nakanishi, Mikael Hartman, Yasushi Yatabe, Ute Hamann, Beth Y Karlan, Suleeporn Sangrajrang, Susanne Krüger Kjaer, Valerie Gaborieau, Allan Jensen, Diana Eccles, Estrid Høgdall, Chen-Yang Shen, Judith Brown, Yin Ling Woo, Mitul Shah, Mat Adenan Noor Azmi, Robert Luben, Siti Zawiah Omar, Kamila Czene, Robert A Vierkant, Børge G Nordestgaard, Henrik Flyger, Celine Vachon, Janet E Olson, Xianshu Wang, Douglas A Levine, Anja Rudolph, Rachel Palmieri Weber, Dieter Flesch-Janys, Edwin Iversen, Stefan Nickels, Joellen M Schildkraut, Isabel Dos Santos Silva, Daniel W Cramer, Lorna Gibson, Kathryn L Terry, Olivia Fletcher, Allison F Vitonis, C Ellen van der Schoot, Elizabeth M Poole, Frans B L Hogervorst, Shelley S Tworoger, Jianjun Liu, Elisa V Bandera, Jingmei Li, Sara H Olson, Keith Humphreys, Irene Orlow, Carl Blomqvist, Lorna Rodriguez-Rodriguez, Kristiina Aittomäki, Helga B Salvesen, Taru A Muranen, Elisabeth Wik, Barbara Brouwers, Camilla Krakstad, Els Wauters, Mari K Halle, Hans Wildiers, Lambertus A Kiemeney, Claire Mulot, Katja K Aben, Pierre Laurent-Puig, Anne Mvan Altena, Thérèse Truong, Leon F A G Massuger, Javier Benitez, Tanja Pejovic, Jose Ignacio Arias Perez, Maureen Hoatlin, M Pilar Zamora, Linda S Cook, Sabapathy P Balasubramanian, Linda E Kelemen, Andreas Schneeweiss, Nhu D Le, Christof Sohn, Angela Brooks-Wilson, Ian Tomlinson, Michael J Kerin, Nicola Miller, Cezary Cybulski, Brian E Henderson, Janusz Menkiszak, Fredrick Schumacher, Nicolas Wentzensen, Loic Le Marchand, Hannah P Yang, Anna Marie Mulligan, Gord Glendon, Svend Aage Engelholm, Julia A Knight, Claus K Høgdall, Carmel Apicella, Martin Gore, Helen Tsimiklis, Honglin Song, Melissa C Southey, Agnes Jager, Ans M Wvan den Ouweland, Robert Brown, John W M Martens, James M Flanagan, Mieke Kriege, James Paul, Sara Margolin, Nadeem Siddiqui, Gianluca Severi, Alice S Whittemore, Laura Baglietto, Valerie McGuire, Christa Stegmaier, Weiva Sieh, Heiko Müller, Volker Arndt, France Labrèche, Yu-Tang Gao, Mark S Goldberg, Gong Yang, Martine Dumont, John R McLaughlin, Arndt Hartmann, Arif B Ekici, Matthias W Beckmann, …, Roger R Reddel, Ellen L Goode, Mark H Greene, Douglas F Easton, Andrew Berchuck, Antonis C Antoniou, Georgia Chenevix-Trench & Alison M Dunning, 27 March 2013, Nature Genetics.
DOI: 10.1038/ng.2566







An excellent article. The case of the sex cells which ought to multiply many more times than an ordinary cell, indeed gets this telomers lengthening strategy to keep itself youthful. You get ovarian cancer and prostrate cancer also side by side, as a result of the knowledge gained by the cancer cells from this trick. The same is the case with the bone marrow which has got a long duty to produce stem cells, pluripotent cells and red carpuscles continuously . It is surprising that mere lengthening strategy of telomers by the enzyme telomerase is not so simple to comprehend and the whole game is so complex . The research in this direction is worth next only to finding God`s particle called Higg’s Boson and also Dark Matter.