DNA is protected by a complex security system involving a double membrane with protein channels. This system prevents unwanted materials from entering while allowing essential messengers to pass through. Credit: Brant M. Webster, et al., doi:10.1016/j.cell.2014.09.012
In a newly published study, Yale biologists and colleagues describe a key quality control mechanism that protects new cells from inheriting defective nuclear pore complexes.
As befitting life’s blueprint, DNA is surrounded by an elaborate security system that assures crucial information is imparted without error.
The security is provided by a double membrane perforated by protein channels that block unwanted material from entering the nucleus and promote entry of key messengers. The breakdown of these channels, called nuclear pore complexes (NPCs), is associated with some forms of cancer and with aging.
Defective nuclear pore complexes (red) are restricted from being inherited by daughter cells demonstrating a quality control pathway during nuclear pore complex biogenesis.
In a new study appearing in the October 9 issue of the journal Cell, Yale researchers Brant Webster, Patrick Lusk, and colleagues describe a key quality control mechanism that protects new cells from inheriting defective NPCs. In the accompanying movie, defective NPCs are sequestered into a specialized compartment (colored red) that is retained in the mother cell, while each daughter inherits functional NPCs. “It is important to understand how these gatekeepers, which are fundamental to cellular function, are built and maintained,” Lusk said.
Abstract:
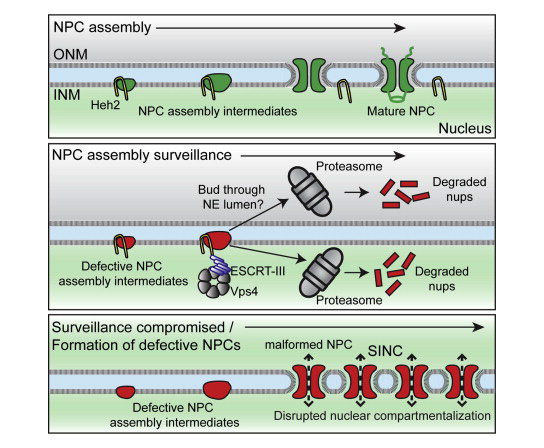
The maintenance of nuclear compartmentalization by the nuclear envelope and nuclear pore complexes (NPCs) is essential for cell function; loss of compartmentalization is associated with cancers, laminopathies, and aging. We uncovered a pathway that surveils NPC assembly intermediates to promote the formation of functional NPCs. Surveillance is mediated by Heh2, a member of the LEM (Lap2-emerin-MAN1) family of integral inner nuclear membrane proteins, which binds to an early NPC assembly intermediate, but not to mature NPCs. Heh2 recruits the endosomal sorting complex required for transport (ESCRT)—III subunit Snf7 and the AAA-ATPase Vps4 to destabilize and clear defective NPC assembly intermediates. When surveillance or clearance is compromised, malformed NPCs accumulate in a storage of improperly assembled nuclear pore complexes compartment, or SINC. The SINC is retained in old mothers to prevent loss of daughter lifespan, highlighting a continuum of mechanisms to ensure nuclear compartmentalization.
Reference: “Surveillance of Nuclear Pore Complex Assembly by ESCRT-III/Vps4” by Brant M. Webster, Paolo Colombi, Jens Jäger and C. Patrick Lusk, 9 October 2014, Cell.
DOI: 10.1016/j.cell.2014.09.012





Be the first to comment on "Yale Biologists Describe DNA’s Security System"