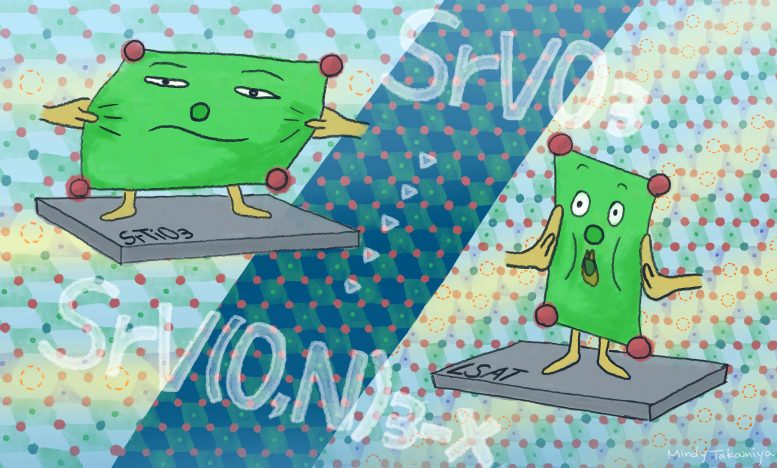
Researchers found a way to create and control the direction and periodicity of the oxygen-vacancy layers in oxynitride crystals at a temperature as low as 600°C (11,00°F). Credit: Mindy Takamiya/Kyoto University iCeMS
Using Strain to Control Oxynitride Properties
A chance discovery leads to a simple process that can introduce ‘oxygen-missing layers’ into perovskite oxynitrides, changing their properties.
Japanese scientists have stumbled onto a simple method for controlling the introduction of defects, called ‘vacancy layers’, into perovskite oxynitrides, leading to changes in their physical properties. The approach, published in the journal Nature Communications, could help in the development of photocatalysts.
Oxynitrides are inorganic compounds formed of oxygen, nitrogen, and other chemical elements. They have gained much attention in recent years because of their interesting properties, with applications in optical and memory devices, and in photocatalytic reactions, for example.
In 2015, solid-state chemist Hiroshi Kageyama of Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS) and his team reported that they found a way to fabricate oxynitrides using a lower temperature ammonia treatment process than the conventional method that requires more than 1,000°C or 1,800°F). The new process produced a polycrystalline powder with layers of missing oxygen atoms, known as oxygen-vacancy planes.
The team wanted to examine the physical properties of this oxynitride, so they grew it as a single crystal thin film on a substrate. “But the oxygen-vacancy layers in the resulting film were in a different plane than the original powder,” Kageyama says. They wondered if the underlying substrate influenced the orientation of the oxygen vacancy layers.
The team grew a film of strontium vanadium oxide (SrVO3) on different substrates and treated it in ammonia at a low temperature of 600°C (11,00°F). The plane of the oxygen vacancy layers and their periodicity — how frequently they appear within the film’s other layers — changed depending on the degree of mismatch between the ‘lattice strains’ in the substrate and the overlying film. Lattice strain is a force applied by the substrate that causes the atoms in a material to be slightly displaced relative to their normal position.
“Even though solid-state chemists have known that oxygen-defect planes play an important role in changing the properties of oxides, such as inducing superconductivity, we haven’t been able to control their formation before,” Kageyama says.
Oxides are typically synthesized using high-temperature reactions, making it difficult to control their crystal structures. Using a lower temperature and strain in this experiment was key for success.
“Our team developed a method to create and control the direction and periodicity of the oxygen-vacancy layers in thin film oxides simply by applying strain,” Kageyama says. “Since the strain energy is enormously large, as large as thousands of degrees Celsius, we’re able to use it to stabilize novel structures that don’t otherwise form.”
Kageyama says it would be interesting to investigate how changes to the thickness of the oxide film, or the reaction temperature and time, could also affect the orientation and periodicity of the oxygen-vacancy layers.
Reference: “Strain-induced creation and switching of anion vacancy layers in perovskite oxynitrides” by Takafumi Yamamoto, Akira Chikamatsu, Shunsaku Kitagawa, Nana Izumo, Shunsuke Yamashita, Hiroshi Takatsu, Masayuki Ochi, Takahiro Maruyama, Morito Namba, Wenhao Sun, Takahide Nakashima, Fumitaka Takeiri, Kotaro Fujii, Masatomo Yashima, Yuki Sugisawa, Masahito Sano, Yasushi Hirose, Daiichiro Sekiba, Craig M. Brown, Takashi Honda, Kazutaka Ikeda, Toshiya Otomo, Kazuhiko Kuroki, Kenji Ishida, Takao Mori, Koji Kimoto, Tetsuya Hasegawa and Hiroshi Kageyama, 23 November 2020, Nature Communications.
DOI: 10.1038/s41467-020-19217-7








Be the first to comment on "A Chance Discovery Leads to a Simple Process That Can Control Perovskite Oxynitride Properties"