
Carbon dioxide (CO2) is a colorless, odorless gas that is essential for life on Earth. It is naturally present in the atmosphere and is important for maintaining the Earth’s temperature through the greenhouse effect. However, excessive amounts of CO2 released by human activities such as burning fossil fuels and deforestation have led to an increase in global temperatures, known as climate change.
The conversion of carbon dioxide (CO2) into carbon monoxide (CO) through electrochemical reactions has the potential to reduce pollution levels by removing CO2 from the atmosphere and also providing an alternative energy source, as CO can be used as an ingredient. However, the catalysts that are currently used in these reactions are not efficient or selective enough to make this process a practical solution.
Now, a team of researchers from the Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences has developed a gold-based hybrid material by modifying gold nanoparticles with a macrocyclic compound called cucurbit[6]uril (CB[6]) that allows for more efficient CO2RR than previously possible.
The results were recently published in the journal Nano Research.
“With this work, we hoped to solve the problem of environmental pollution and energy shortage through electrochemical conversion of carbon dioxide to value-added products,” said corresponding author Minna Cao of the State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, and of the University of Chinese Academy of Sciences. “In order to enhance the local CO2 concentration on the catalysts’ surface, we utilize macromolecule cucurbit[n]uril to functionalize gold surface, which is the distinguishing feature of our work from those that have been done before.”
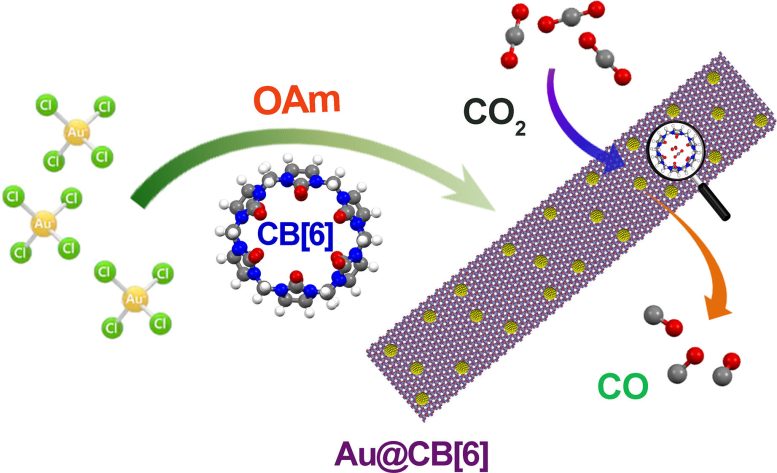
A gold-based hybrid material (Au@CB[6]) is modified by CB[6] in order to efficiently convert CO2 to CO. Credit: Nano Research, Tsinghua University Press
The researchers created a controlled synthesis of nanoparticles by modifying CB[6]. CB[6] has negatively charged portals and positively charged surfaces, which helps contribute to the result of having the electronic interaction between CB[6] and metal regulate the catalytic performance.
The researchers verified both the morphology and surface structure of the nanoparticles through transmission electron microscopy. The gold-based hybrid material (Au@CB[6]) was proven to enhance CO2RR catalytic activity.
“We have proved the interaction between cucurbit[6]uril and CO2 through operando electrochemical measurement and density functional theory calculations,” Cao said.
According to the researchers, multiple factors contribute to the improved catalytic performance. First, the CB[6] can increase the local CO2 concentration near the metal surface by gathering CO2, which means that the Au@CB[6] has tunable, or adjustable, CO2 enrichment. Additionally, the modification of CB[6] allows for improved CO2RR by breaking the previously mentioned scaling relations of the binding affinity between the catalyst surface and CO2/CO.
Also, one reason that CO2RR was limited in efficiency with gold surface catalysts previously is that CO2 has low solubility in aqueous electrolytes, an issue that the researchers solved by using the highly specific binding force of macrocycle to selectively adsorb certain species to regular the electrocatalytic reaction.
“The results showed that CB[6] can gather CO2 and lead the increased local CO2 concentration near the metal interface, as well as promote CO desorption, which are the dominating reasons for enhanced CO2RR performance,” Cao said. “Using the rigid macrocycle cucurbit[n]uril to modify the catalysts’ surface is a promising pathway to enhance the electrocatalytic performance.”
The researchers plan to continue to modify the catalyst in order to further improve the efficiency of the CO2RR.
“In the next step, we hope to adjust the shape and size of the gold catalyst in the presence of cucurbit[n]uril to further promote the catalytic performance toward electrochemical reduction of carbon dioxide to value-added products,” Cao said.
Reference: “Tunable CO2 enrichment on functionalized Au surface for enhanced CO2 electroreduction” by Huimin Wang, Yuqing Fu, Zhe-Ning Chen, Wei Zhuang, Minna Cao and Rong Cao, 5 December 2022, Nano Research.
DOI: 10.1007/s12274-022-5159-8
The research was funded by the National Key R&D Program of China, the NSFC, and the Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China.








Oh thank God, carbon monoxide! Now we can all die sooner by converting CO2 into an actual toxic pollutant, and not have to read ClimateChangeDaily articles, at least for a month before it naturally turns back into CO2 in the atmosphere.
Exactly! Carbon Monoxide is a killer of Humans.
Uh yeah, you use the regas (CO) to make whatever feedstocks you want so the carbon stays fixed. Try for diamond or diamondene, be thrilled if it’s ethylene oxide, or just treat compressed timber until it’s even more inured to heat. You could even get antioxidants like NADP+ or carotene but it seems like that might be a 3 step process or more?
Gold x turmeric is canny too though, right? Next they’ll combine ginkgo heartwood and flax oil with it and get [has to look] even better reduction reaction to valuable product efficiency…maybe hold the monoxide, as you say. Varying the gold NP sounds easy though eh?
Yes, that’s nice. But haven’t you ever asked yourself from where that temperature has came except the sun light. How much impact on global warming have human made heat sources – all of their kind. How much heat energy is thrown daily just with each car, house heating, all kind of thermal power plants, plains trains, ships etc… Is there anyone calculated those heat sources… It’s already long time known that average temperature in cities is at least 2-3°C bigger than in fields or woods just few kilometres away of city? Isn’t it something wrong with CO2 only – global warming concept?
Enlightenment would be nice… Because I feel that there something smells strange, I mean all that story about CO2 and global warming.
You can do a rough estimate yourself using freshman physics. Many of the molecules we use for fuel and whatnot were synthesized with the help of solar energy, effectively sequestering that energy, preventing it from directly impacting global temperature. Burning CO2-based fuel by driving a car produces heat directly due to the inefficiency of converting the released energy into mechanical motion (friction). You can calculate how much mechanical heat is produced in this way, and compare it to how much additional infrared radiation from normal solar heating of the Earth will be prevented from escaping the atmosphere, thus increasing the Earth’s temperature.
You could include other variables in your model, such as naturally occurring carbon sinks, their efficiency, capacity and such, but to keep it simple for now, just comparing the two processes, friction vs greenhouse effect, would indicate why Global Warming from the deliberate release of CO2 into the atmosphere is such a serious problem.
“However, excessive amounts of CO2 released by human activities such as burning fossil fuels and deforestation have led to an increase in global temperatures, known as climate change.”
It used to be known as global warming until the climate started cooling again and the total amount of warming is still less than about one degree C. in spite of an excessive” 50% increase in CO2.
In order to have any meaningful effect on the Earth’s climate any technologies (all combined) would have to remove and store durably billions of tons of CO2…and do it without adding more in the process. That’s a big hurdle to overcome, never mind the costs involved.
So we can turn CO2 into CO and then burn it to CO2? Wonderful.
Ignore that energy hiding behind the curtain! Rube Goldberg would approve.
Plant some around the 20 or so volcanos going off at any moment around the world. There must be a lot of stupidity among the know-it-alls that figure MAN can stop the CO2 Rush.
STOP trying to make it EASIER for morons to pollute the planet.
Try instead to make it painful. morons.
goooooooooooooooood
gooooood
The earth is not a closed system.
Therefore the experiment does not apply.
“The earth is not a closed system.”
Jesus weeps over these comments. For all intents and purposes of this discussion it is.