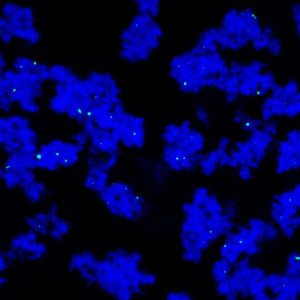
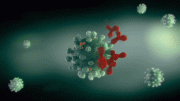
A novel separation technique uses starch-coated magnetic nanoparticles (green) and polyethylene glycol to purify monoclonal antibodies (blue). Credit: 2013 Elsevier
Researchers from A*STAR have developed a high-capacity method to purify monoclonal antibodies that uses magnetic nanoparticles.
Monoclonal antibodies represent the largest and fastest-growing segment of international biopharma. While these therapeutic agents are a boon for global healthcare, productivity constraints pose a serious challenge for manufacturers seeking to make sufficient amounts for therapeutic applications. Now, A*STAR researchers have developed a high-capacity method to purify monoclonal antibodies that uses magnetic nanoparticles and also introduces new operating conditions.
At present, therapeutic antibodies are generally purified by a technique known as protein A affinity chromatography. The process yields a high purification factor — typically 99 percent — but it is slow, thereby creating a severe productivity bottleneck. The process is largely hindered by the low capacity of protein A, which binds monoclonal antibodies at an average rate of 50 grams per liter of protein A chromatography media. The overall purification process requires unpurified antibodies to pass through columns packed with the media in multiple cycles that can take up to a week.
A research team led by Pete Gagnon and co-workers from the A*STAR Bioprocessing Technology Institute in Singapore have developed an alternative method with 1,000 times the capacity of protein A. The technique involves the use of polyethylene glycol, which causes the antibodies to be deposited on the surface of starch-coated magnetic nanoparticles. The particles are collected in a magnetic field, undeposited contaminants are washed away and the purified antibodies recovered by removing the polyethylene glycol.
“The high capacity of our nanoparticle method makes it much faster than column chromatography,” explains Gagnon. “Instead of the pharmaceutical industry norm of five to eight cycles, the new process requires only one cycle, which takes just a few hours.” This reduction dramatically increases the productivity of the new approach over traditional methods.
The new method also required the research team to develop new operating conditions. Polyethylene glycol has been used for decades to process antibodies, but it has never achieved the level of purity needed for clinical therapeutics. The team discovered that by elevating the salt concentration, they could reduce contaminant levels from about 250,000 parts per million to 500: the same level achieved by protein A. A single follow-on polishing step using a multimodal chromatography column further purified the antibodies to clinical quality standards.
Gagnon notes the high potential for adoption of the new technology by industry. In addition to solving the long-standing problem of productivity for monoclonal antibodies, the nanoparticle approach can be applied to many other therapeutic proteins and also to viral vaccines.
The A*STAR-affiliated researchers contributing to this research are from the Bioprocessing Technology Institute.
Reference: “High productivity purification of immunoglobulin G monoclonal antibodies on starch-coated magnetic nanoparticles by steric exclusion of polyethylene glycol” by Pete Gagnon, Phyllicia Toh and Jeremy Lee, 25 November 2013, Journal of Chromatography A.
DOI: 10.1016/j.chroma.2013.11.039




Be the first to comment on "A*STAR Researchers Develop High-Capacity Method to Purify Monoclonal Antibodies"