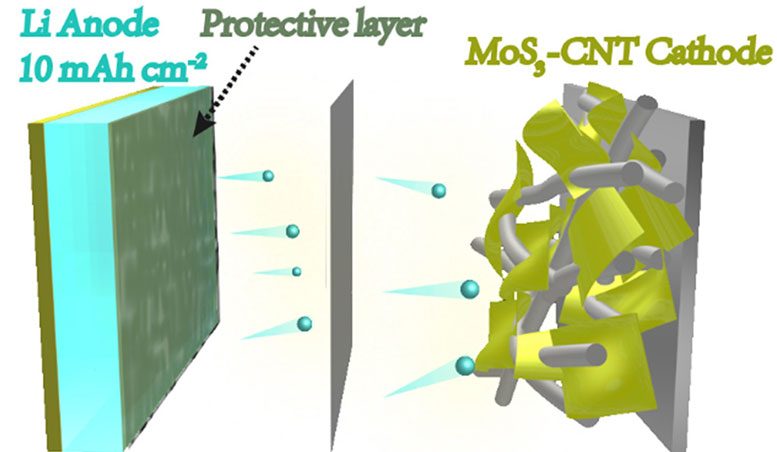
This image shows the schematic structure of a new battery cell with lithium metal electrodes developed at Yale and Donghua University.
Researchers at Yale and Donghua University in China have developed a new process for creating lithium metal that may boost the energy and capacity of rechargeable batteries.
Lithium metal is considered the best option as a material for anodes in high-energy batteries, the researchers said, because of the metal’s high potential for providing large amounts of energy and capacity in a given mass. Yet existing lithium metal electrodes, limited by low capacity and utilization efficiency, have not come close to reaching that potential.
In a new study on May 14 in the journal Proceedings of the National Academy of Sciences, a team led by Yale’s Hailiang Wang describes a new approach to creating more efficient lithium metal electrodes. The process yields a protective layer that enables lithium metal anodes to be efficiently discharged and charged at high capacities.
Based on the new process, the researchers constructed a battery cell that outperforms other laboratory-scale battery cells, as well as state-of-the-art lithium-ion batteries on the market.
Hailiang Wang is an assistant professor of chemistry and a member of the Energy Sciences Institute at Yale’s West Campus. The first author of the study is Qiuwei Shi, a graduate student at Donghua and visiting student at Yale. Additional authors are Yiren Zhong and Min Wu from Yale and Hongzhi Wang from Donghua.
Reference: “High-capacity rechargeable batteries based on deeply cyclable lithium metal anodes” by Qiuwei Shi, Yiren Zhong, Min Wu, Hongzhi Wang and Hailiang Wang, 14 May 2018, PNAS.
DOI: 10.1073/pnas.1803634115







Be the first to comment on "Chemists Develop New Way to Create Lithium Metal Electrodes for Batteries"