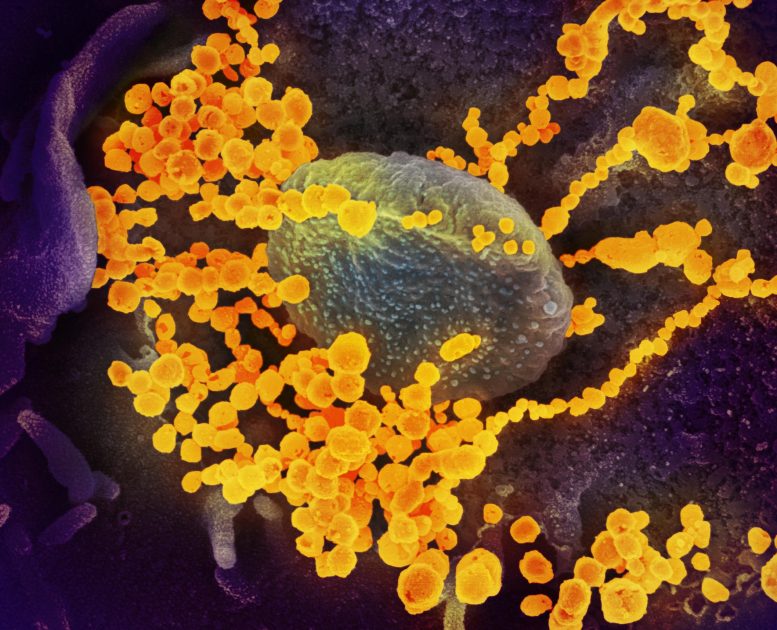
This scanning electron microscope image shows SARS-CoV-2 (round gold objects) emerging from the surface of cells cultured in the lab. SARS-CoV-2, also known as 2019-nCoV, is the virus that causes COVID-19. The virus shown was isolated from a patient in the U.S. Credit: NIAID-RML
Study Supports Clinical Testing Under Way Across U.S.
Early treatment with the experimental antiviral drug remdesivir significantly reduced clinical disease and damage to the lungs of rhesus macaques infected with SARS-CoV-2, the coronavirus that causes COVID-19, according to National Institutes of Health scientists.
The study was designed to follow dosing and treatment procedures used for hospitalized COVID-19 patients being administered remdesivir in a large, multi-center, clinical trial led by NIH’s National Institute of Allergy and Infectious Diseases (NIAID). The scientists posted the work on the preprint server bioRxiv. The findings are not yet peer-reviewed and should not be considered clinical advice, but are being shared to assist the public health response to COVID-19. A study detailing the development of the rhesus macaque model of mild- to-moderate human disease, conducted by the same team of NIAID scientists, was posted to bioRxiv on March 21.
The current study of remdesivir, a drug developed by Gilead Sciences Inc. and NIAID-supported investigators, involved two groups of six rhesus macaques. One group of monkeys received remdesivir and the other animals served as an untreated comparison group. Scientists infected both groups with SARS-CoV-2. Twelve hours later the treatment group received a dose of remdesivir intravenously, and then received a daily intravenous booster dose thereafter for the next six days. The scientists timed the initial treatment to occur shortly before the virus reached its highest level in the animals’ lungs.
Twelve hours after the initial treatment, the scientists examined all animals and found the six treated animals in significantly better health than the untreated group, a trend that continued during the seven-day study. They report that one of the six treated animals showed mild breathing difficulty, whereas all six of the untreated animals showed rapid and difficult breathing. The amount of virus found in the lungs was significantly lower in the treatment group compared to the untreated group, and SARS-CoV-2 caused less damage to the lungs in treated animals than in untreated animals.
The investigators note that the data supports initiating remdesivir treatment in COVID-19 patients as early as possible to achieve maximum treatment effect. The authors, from NIAID’s Rocky Mountain Laboratories in Hamilton, Montana, also note that while remdesivir helped prevent pneumonia, it did not reduce virus shedding by the animals. “This finding is of great significance for patient management, where a clinical improvement should not be interpreted as a lack of infectiousness,” they write.
Reference: “Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2” by Brandi N. Williamson, Friederike Feldmann, Benjamin Schwarz, Kimberly Meade-White, Danielle P. Porter, Jonathan Schulz, Neeltje van Doremalen, Ian Leighton, Claude Kwe Yinda, Lizzette Pérez-Pérez, Atsushi Okumura, Jamie Lovaglio, Patrick W. Hanley, Greg Saturday, Catharine M. Bosio, Sarah Anzick, Kent Barbian, Tomas Cihlar, Craig Martens, Dana P. Scott, Vincent J. Munster and Emmie de Wit, 21 March 2020, bioRxiv.
DOI: 10.1101/2020.04.15.043166





Be the first to comment on "Clinical Test Results Released for Antiviral Remdesivir Treatment of COVID-19"