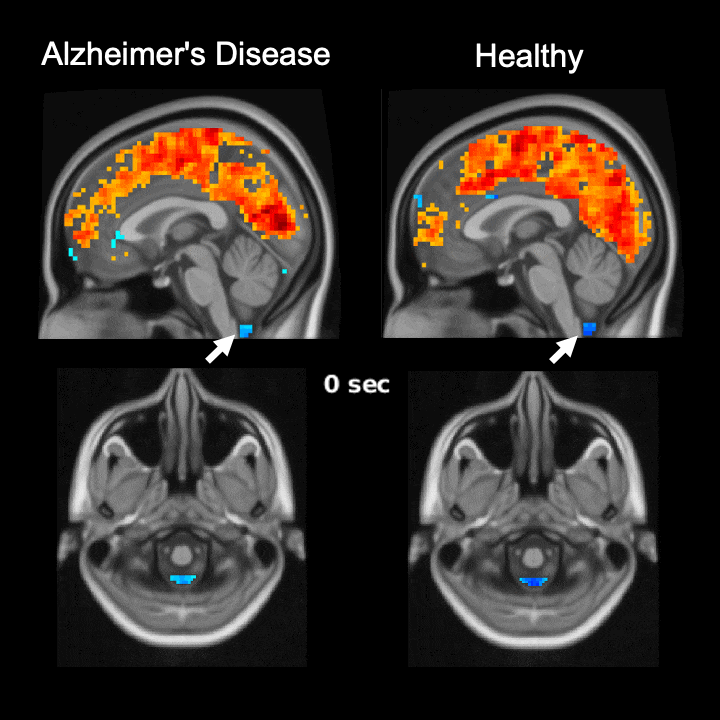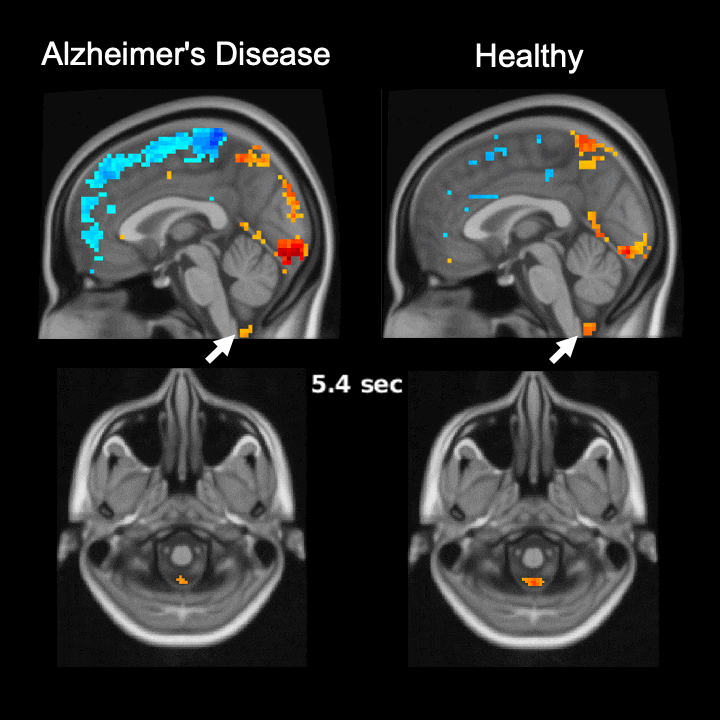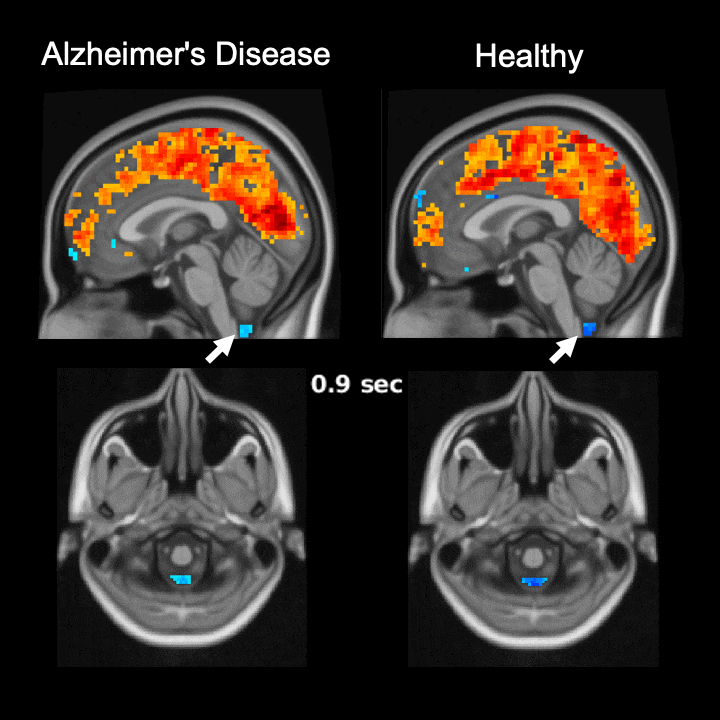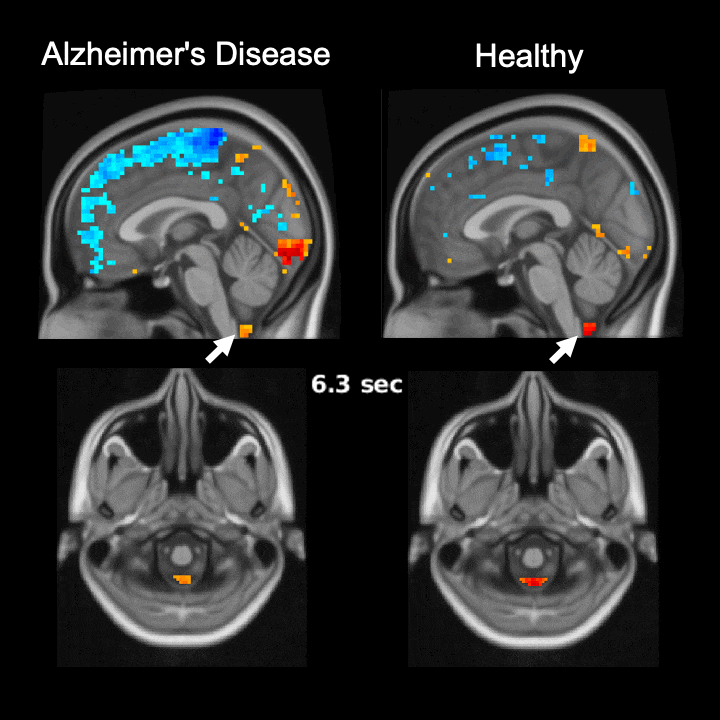
The global brain signal in Alzheimer’s disease patients is associated with weaker cerebrospinal fluid flow as compared with healthy controls. Credit: Feng Han and Xiao Liu @ The Pennsylvania State University
Global brain activity seen on fMRI, and its connection with cerebrospinal fluid flow weaker in brains of individuals with Alzheimer’s disease risk or related toxin buildup.
Evidence of sleep-dependent low-frequency (<0.1 Hz) global brain activity in the clearance of Alzheimer’s disease-related toxin buildup is presented in research published today (June 1, 2021) in the open access journal PLOS Biology by Xiao Liu and colleagues at The Pennsylvania State University. This neuronal activity was more strongly linked with cerebrospinal fluid flow in healthy controls than higher risk groups and patients, and the findings could serve as a potential imaging marker for clinicians in evaluating patients.
The development of Alzheimer’s disease is believed to be driven by the buildup of the toxic proteins amyloid-β and tau in the brain. The brain’s glymphatic system plays a crucial role in clearing these toxins and previous work has shown a possible relationship between sleep-dependent global brain activity and the glymphatic system by showing this activity is coupled by cerebrospinal fluid flow essential for the glymphatic system.
Using 118 subjects in the Alzheimer’s Disease Neuroimaging Initiative project, the researchers measured this global brain activity and cerebrospinal fluid flow as well as looking at behavioral data. Individuals underwent resting-state fMRI sessions two years apart, and the team compared their findings with neurobiological and neuropsychological markers related to Alzheimer’s disease, such as levels of the toxic protein amyloid-β.
The strength of the connection between brain activity and cerebrospinal fluid flow was weaker in individuals at a higher risk or who had already developed Alzheimer’s disease. Additionally, this weaker connection was associated with higher levels of amyloid-β and disease-related behavioral measures two years later. This suggests an important role for sleep-dependent global brain activity in the clearance of brain waste, and its connection to cerebrospinal fluid flow could be helpful as a future marker for clinical evaluation.
Dr. Liu adds, “The study linked the coupling between the resting-state global brain activity and cerebrospinal fluid flow to Alzheimer’s disease pathology. The finding highlights the potential role of low-frequency (<0.1 Hz) resting-state neural and physiological dynamics in the neurodegenerative diseases, presumably due to their sleep-dependent driving of cerebrospinal fluid flow to wash out brain toxins. Future studies are warranted to fully understand the global brain activity and associated physiological modulations and their role in glymphatic clearance and neurodegenerative diseases.”
Reference: “Reduced coupling between cerebrospinal fluid flow and global brain activity is linked to Alzheimer disease-related pathology” by Feng Han, Jing Chen, Aaron Belkin-Rosen, Yameng Gu, Liying Luo, Orfeu M. Buxton, Xiao Liu and the Alzheimer’s Disease Neuroimaging Initiative, 1 June 2021 PLOS Biology.
DOI: 10.
Funding: This work is supported by funding from the National Institutes of Health Pathway to Independence Award (K99/R00 5R00NS092996-03 to X.L.), the Brain Initiative award (1RF1MH123247-01 to X.L.), the NIH R01 award (1R01NS113889-01A1 to X.L.), and NIA R03 award (1R03AG070812-01 to L.L.). O.M.B. received subcontract grants to Penn State from Proactive Life LLC (formerly Mobile Sleep technologies) doing business as SleepScape (NSF/STTR #1622766, NIH/NIA SBIR R43- AG056250, R44-AG056250), received honoraria/travel support for lectures from Boston University, Boston College, Tufts School of Dental Medicine, New York University and Allstate, and receives an honorarium for his role as the Editor in Chief of Sleep Health. Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904; Principal Investigator: Michael Weiner) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012; Principal Investigator: Michael Weiner). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.









neuology
A 44 Vancouver St.
yarmouth, n.s.
b5a 2p3
I wonder what the implications are for Idiopathic intercranial hypertension / Psuedotumor Cerebri patients? Are we at higher risk of Alzheimer’s?