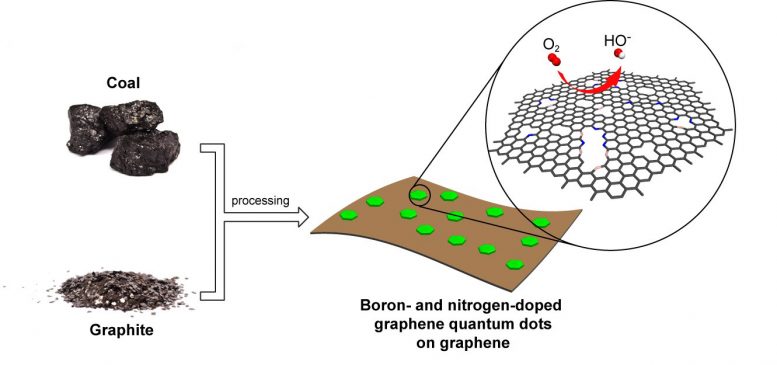
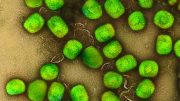
Rice University scientists combined graphene quantum dots, graphene oxide, nitrogen, and boron into a catalyst capable of replacing platinum in fuel cells at a fraction of the cost. Credit: Tour Group/Rice University
Researchers at Rice University have developed a cheap hybrid catalyst made from graphene quantum dots that outperforms platinum catalysts for certain reactions within fuel cells.
Graphene quantum dots created at Rice University grab onto graphene platelets like barnacles attach themselves to the hull of a boat. But these dots enhance the properties of the mothership, making them better than platinum catalysts for certain reactions within fuel cells.
The Rice lab of chemist James Tour created dots known as GQDs from coal last year and have now combined these nanoscale dots with microscopic sheets of graphene, the one-atom-thick form of carbon, to create a hybrid that could greatly cut the cost of generating energy with fuel cells.
The research is the subject of a new paper in the American Chemical Society journal ACS Nano.
The lab discovered boiling down a solution of GQDs and graphene oxide sheets (exfoliated from common graphite) and combined them into self-assembling nanoscale platelets that could then be treated with nitrogen and boron. The hybrid material combined the advantages of each component: an abundance of edges where chemical reactions take place and excellent conductivity between GQDs provided by the graphene base. The boron and nitrogen collectively add more catalytically active sites to the material than either element would add alone.
“The GQDs add to the system an enormous amount of edge, which permits the chemistry of oxygen reduction, one of the two needed reactions for operation in a fuel cell,” Tour said. “The graphene provides the conductive matrix required. So it’s a superb hybridization.”
The Tour lab’s material outperformed commercial platinum/carbon hybrids commonly found in fuel cells. The material showed an oxygen reduction reaction of about 15 millivolts more in positive onset potential – the start of the reaction – and 70 percent larger current density than platinum-based catalysts.
The materials required to make the flake-like hybrids are much cheaper, too, Tour said. “The efficiency is better than platinum in terms of oxygen reduction, permitting one to sidestep the most prohibitive hurdle in fuel-cell generation — the cost of the precious metal,” he said.
Rice graduate student Huilong Fei is the paper’s lead author. Co-authors are graduate students Ruquan Ye, Gonglan Ye, Yongji Gong, Zhiwei Peng, and Errol Samuel; research technician Xiujun Fan; and Pulickel Ajayan, the Benjamin M. and Mary Greenwood Anderson Professor in Mechanical Engineering and Materials Science and of chemistry and chair of the Department of Materials Science and NanoEngineering, all of Rice.
Tour is the T.T. and W.F. Chao Chair in Chemistry as well as a professor of materials science and nanoengineering and of computer science.
The Office of Naval Research Multidisciplinary University Research Initiative (MURI) program, the Air Force Office of Scientific Research, and its MURI program supported the research.
Reference: “Boron- and Nitrogen-Doped Graphene Quantum Dots/Graphene Hybrid Nanoplatelets as Efficient Electrocatalysts for Oxygen Reduction” by Huilong Fei, Ruquan Ye, Gonglan Ye, Yongji Gong, Zhiwei Peng, Xiujun Fan, Errol L. G. Samuel, Pulickel M. Ajayan and James M. Tour, 24 September 2014, ACS Nano.
DOI: 10.1021/nn504637y



Be the first to comment on "Graphene Quantum Dots Outperform Platinum in Fuel Cells"