
Professor Shai Rahimipour from the Chemistry Department at Bar-Ilan University in Israel has adopted a distinct approach in his pursuit of a drug treatment for Alzheimer’s disease. By utilizing theranostics to identify and treat the initial pre-symptomatic indications of the disease, Rahimipour aims to prevent the irreversible brain cell damage that occurs as the disease progresses. His innovative approach shows promise in stopping the advancement of the disease and has gained substantial recognition in the scientific community.
A new molecule developed by researchers at Bar-Ilan University has proven effective in diagnosing Alzheimer’s in its earliest pre-symptomatic stages and in preventing progression of the disease.
More than 55 million people worldwide were living with Alzheimer’s disease in 2020, according to Alzheimer’s Disease International. This figure is expected to almost double every 20 years, reaching 78 million in 2030 and 139 million in 2050. In 2021 the WHO Global Status Report estimated the annual worldwide cost of dementia as over $1.3 trillion and anticipated to rise to $2.8 trillion by 2030.
To date, most drugs developed to treat Alzheimer’s disease have failed, largely because they target the wrong biomarkers and individuals already exhibiting signs of the disease. Once symptoms appear, however, many brain cells responsible for memory and cognition are likely already damaged and beyond repair.
Professor Shai Rahimipour in the Chemistry Department at Bar-Ilan University in Israel has pioneered a different approach utilizing theranostics to pinpoint and treat the earliest, pre-symptomatic signs of Alzheimer’s disease. Showing promise in stopping the progression of the disease before onset of irreversible brain cell damage, Rahimipour’s groundbreaking approach has garnered significant attention in the scientific world.
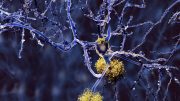
In Alzheimer’s disease, a small protein known as amyloid beta misfolds to intermediates that aggregate into larger macromolecular structures known as fibrils and plaques.
Because plaques are visible under a microscope, scientists long believed that they are responsible for damaging neurons in Alzheimer’s disease etiology. Many clinical trials and billions of dollars were invested over more than a quarter of a century to generate molecules and antibodies targeting and preventing formation of fibrils and plaques. Such treatments proved unsuccessful and caused intolerable side effects. Over time, fibrils and plaques themselves were deemed non-toxic, and instead earlier soluble intermediates known as oligomers are now considered the culprits in this insidious disease.
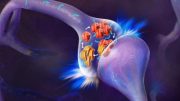
Recent clinical trials using antibodies to target oligomers have shown promising results, and the Biogen/Essai antibodies Aducanumab and Lecanemab have received US Food and Drug Administration (FDA) approval. Controversy over efficacy and notable side effects such as microhemorrhages and brain swelling highlight the need for better therapy and tools for early Alzheimer’s disease detection to improve standard of care. Moreover, most antibodies don’t reach the brain sufficiently because the blood-brain barrier limits penetration of proteins and antibodies.
Rahimipour and his team have overcome these barriers by developing small abiotic and drugable cyclic peptides that have proven effective in animal models in diagnosing early pre-symptomatic stage of Alzheimer’s and treating the disease by targeting oligomers. When these molecules were combined in a test tube with the small protein amyloid beta, the generation of oligomers was completely blocked, and no subsequent aggregation occurred.
In the next stage, the researchers incubated human neurons with the toxic oligomers and the cyclic peptides. Most neurons remained alive, but those in the control group that were exposed to the oligomers without cyclic peptides were severely damaged and died.
Next, they tested the efficacy of the cyclic peptides in transgenic C. elegans worms that develop symptoms like those in Alzheimer’s disease. The researchers observed that feeding the worms with the cyclic peptides dramatically extended the survival of the worms and abolished the appearance of the disease by preventing the formation of early toxic oligomers, suggesting that the aggregation process can be stopped in the very early stages of the disease, even before oligomers are formed.
The researchers then examined transgenic mice using a radioactive version of the cyclic peptides to obtain a pre-symptomatic diagnosis through Positron Emission Tomography (PET), a technique commonly used in hospitals. Much to their delight, the molecule detected for the first time early amyloid beta oligomers in the thalamus (which relays motor and sensory signals to the cerebral cortex) of pre-symptomatic mice prior to their spread to other brain parts. That is, they successfully predetermined the onset of the disease before the formation of amyloid fibrils and plaques, and before the appearance of symptoms of Alzheimer’s disease!
Next, the transgenic mice in the pre-symptomatic stage were treated with the cyclic peptides and observed over time for memory functions and amount of amyloid beta oligomers in the brain. Through molecular imaging, the researchers determined that the mice didn’t generate substantial amounts of oligomers and, consequently, didn’t develop any sign of Alzheimer’s.
“In these animal models, we have, in effect, halted the disease in its early stages, even before oligomers are formed. One great advantage of our synthetic molecules, in contrast to natural antibodies, is that they are not immunogenic, and they remain in the body much longer, so fewer injections or applications are likely needed,” says Prof. Rahimipour. “Our meticulous regime of experiments has shown no sign of toxicity and that, unlike antibodies, the molecules cross the blood-brain barrier very well,” he adds.
Prof. Rahimipour’s research was recently published in the journal Proceedings of the National Academy of Sciences, in collaboration with colleagues from the Université de Sherbrooke and the Université de Montréal in Canada. He is now working on the development of an appropriate drug for pre-clinical and clinical trials.
Reference: “Early diagnosis and treatment of Alzheimer’s disease by targeting toxic soluble Aβ oligomers” by Maram Habashi, Suresh Vutla, Kuldeep Tripathi, Sudipta Senapati, Pradeep S. Chauhan, Anat Haviv-Chesner, Michal Richman, Samia-Ait Mohand, Véronique Dumulon-Perreault, Ramakotaiah Mulamreddy, Eitan Okun, Jordan H. Chill, Brigitte Guérin, William D. Lubell and Shai Rahimipour, 28 November 2022, Proceedings of the National Academy of Sciences.
DOI: 10.1073/pnas.2210766119



Good News! My husband has diminishing cognitive function and it finally sounds like there is some hope.
Great news problem being how long before this drug will be available it’s a devastating disease.
All of the compounds developed prevented amyloid plaques in mouse models but were ineffective in people. many things work in mice / rats but failing the clinic