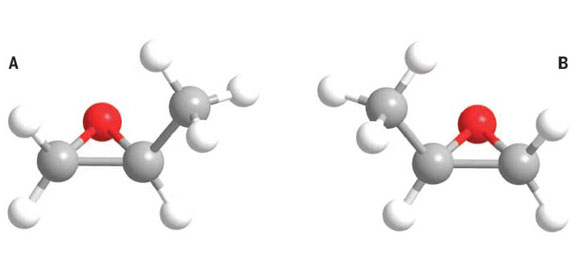
The molecular structure of S-propylene oxide (A) and R-propylene oxide (B). Carbon, hydrogen, and oxygen atoms are indicated by gray, white, and red spheres, respectively.
A team of astronomers from the Harvard-Smithsonian Center for Astrophysics has detected chiral molecules in interstellar space. Life on Earth is based on chiral molecules.
Chiral molecules are those that have a “handedness,” that is, they take one of two forms, identical in chemical composition but with mirror image shapes – like our right and left hands: no rotation can convert one into the other. For reasons that are not known, essentially all biological molecules are homochiral (having only one shape). On Earth this shape is left-handed; our bodies cannot digest right-handed sugars.
The origin of homochirality is a key mystery in the study of our cosmic origins. Although some scientists have argued it has some evolutionary advantages, the mechanisms for the selection of one shape over another are uncertain. Disentangling them requires that we understand the potential origins of the phenomenon. The oldest materials studied in the laboratory so far are meteoritic samples, but astronomers have been searching for even more ancient examples in the rich chemistry of the interstellar medium using techniques of radio astronomy to look for spectral signatures of gaseous chiral molecules.
CfA astronomer Ryan Loomis was part of a team of scientists who have detected the first known chiral, interstellar molecule, propylene oxide (CH3CHCH2O). The scientists predicted the strength of its spectral features using their models, and identified three features that they suspected would be strong enough to detect. One feature lies in a part of the radio spectrum with high background noise, but the other two, with wavelengths of about two centimeters, are in the clear. They searched for these spectral signatures in the public archives of the Green Bank Telescope’s observations of the giant molecular cloud Sagittarius B2, which is famous for its wealth of complex molecules. They clearly detected each of them, and for good measure, they sought and found the third line using the Australian Parkes Radio Telescope which operates in a much lower background noise environment. A careful study of other possible explanations for the absorption features found no other reasonable candidates.
Both chiralities have identical spectra, and so although the new results may have detected ancient, chiral molecules it is not known if they are homochiral. Nor do these results identify any mechanism that might eventually preferentially select one handedness over the other. However, the team can calculate the likely environment of these molecules, which is in a cold, shocked shell surrounding a dense core with many other organic species. They speculate briefly on the role of one particularly interesting possibility: circularly polarized light in the cloud, which also has a handedness. More research is clearly needed; meanwhile, the new results demonstrate that chiral molecules exist in space and are ready for study.
Reference: “Discovery of the interstellar chiral molecule propylene oxide (CH3CHCH2O)” by Brett A. Mcguire, P. Brandon Carroll, Ryan A. Loomis, Ian A. Finneran, Philip R. Jewell, Anthony J. Remijan and Geoffrey A. Blake, 17 Jun 2016, Science.
DOI: 10.1126/science.aae0328








I thought we were mostly right rotating and not left rotating? Dextrose is edible. Another dyslexic writer?
Yes, this is a funny mistake.
Dextrose is the name for edible Glucose – Dexter (or Rectus) means Right while Sinister means Left. So our bodies CAN digest right-handed sugar called Dextrose.
Just to avoid even more confusion of other readers the D-(R) and S-(L) are assigned to chiral molecule “versions” (aka optical isomers) based on arbitrary rule: how atoms are “rotating” ordered by their atomic numbers around the considered stereocenter of the molecule (and other ~CIP rules).
It is not an absolute feature with any specific generally valid meaning from which we could say “whatever left-handed molecule is better for this or that” or that “we were right rotating”.
We can only say that for specific molecules and only in context of other specific chirality of other molecules.
D-WhateverX reacts better with D-WhateverY than with S-WhateverY, still it reacts better with S-WhateverZ than with D-WhateverZ.
Specifically it is important in relation to the homochiral biochemistry molecules.
In this article scientists have NO CLUE what is the detected molecule chirality, though this is the most interesting feature to detect (although a speculation has been mentioned).