
Furthermore, the researchers found that connective tissue cells play a significant role in heart regeneration by temporarily entering an activated state.
Zebrafish may repair heart tissue after injury, according to research conducted by the MDC team under the direction of Jan Philipp Junker and Daniela Panáková.
When a person has a heart attack and does not get prompt treatment, the heart muscle cells (cardiomyocytes) are damaged by a lack of oxygen and begin to die. Scar tissue grows, and since we can’t make new cardiomyocytes, the heart can’t pump as effectively as it should. However, things are radically different for lower vertebrates such as the zebrafish, which can regenerate organs including its heart.
“We wanted to find out how this little fish does that, and if we could learn from it,” says Professor Jan Philipp Junker, head of the Quantitative Developmental Biology Lab at the Berlin Institute for Medical Systems Biology (BIMSB), part of the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) in Berlin.
The researchers simulated myocardial infarction injuries in the hearts of their zebrafish with the help of Dr. Daniela Panáková, who leads the Electrochemical Signaling in Development and Disease Lab at the MDC. They monitored the regeneration of the cardiomyocytes using single-cell analyses and cell lineage trees. Their findings have recently been published in Nature Genetics.
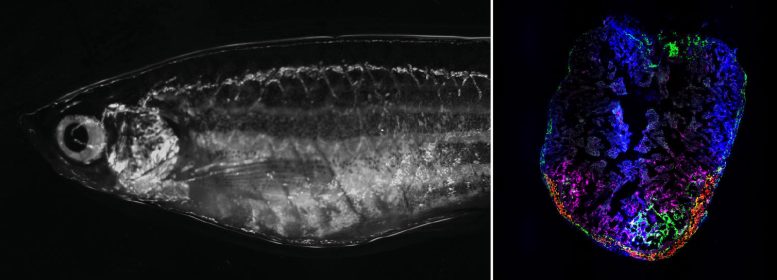
Right: Adult zebrafish in the brightfield microscope. Left: Zebrafish heart 7 days after cryoinjury. Transiently activated fibroblasts localize to the injury area. Credit: Panáková Lab, MDC
Human hearts stop short of regeneration
The zebrafish’s one-millimeter-sized heart was exposed to a cold needle for a few seconds by the researchers while they observed it under a microscope. Any tissue the needle touched died. Similar to those who have had a heart attack, this results in an inflammatory response, which is followed by fibroblast-produced scarring.
“Surprisingly, the immediate response to the injury is very similar. But while the process in humans stops at that point, it carries on in the fish. They form new cardiomyocytes, which are capable of contracting,” says Junker.
“We wanted to identify the signals that come from other cells and help drive the regeneration,” he continues. Junker’s team used single-cell genomics to search the injured heart for cells that don’t exist in a healthy zebrafish heart.
Three new fibroblast types that momentarily become activated were discovered by the researchers. Despite sharing an appearance with other fibroblasts, these activated cells have the ability to read a variety of additional genes that are involved in the formation of proteins, such as connective tissue factors like collagen 12.
Fibroblasts give the signal for regeneration
In humans, fibrosis, also known as scarring, is thought to be a barrier to heart regeneration. However, once activated, the fibroblasts seem to be crucial to the process. When Panáková used a genetic trick to turn off the collagen 12-expressing fibroblasts in the zebrafish, it became evident just how crucial they are. The result: no regeneration. Junker believes it makes sense that fibroblasts are responsible for giving the repair signals: “They form right at the site of injury, after all,” he says.
To identify the source of these activated fibroblasts, Junker’s team produced cell lineage trees using a technique called LINNAEUS, which his lab developed in 2018. LINNAEUS works with genetic scars that collectively act like a barcode for the origin of every cell.
“We create this barcode using CRISPR-Cas9 genetic scissors. If, after injury, two cells have the same barcode sequence, it means they’re related,” explains Junker.
The researchers identified two sources of temporarily activated fibroblasts: the outer layer of the heart (epicardium) and the inner layer (endocardium). Cells producing collagen 12 were found exclusively in the epicardium.
Different disciplines worked closely on the study
Multiple MDC researchers collaborated throughout the study – from the experiments on the fish, to the genetic analyses, to the bioinformatic interpretation of the results.
“For me, the most exciting thing was to see how well our disciplines complement each other and how we could verify results from bioinformatics on a living animal,” says Sara Lelek, who is a lead author of the study and was responsible for the animal tests. “It was a big project that allowed us all to contribute our expertise. I think that’s why the study is so comprehensive and so useful for many researchers.”
Dr. Bastiaan Spanjaard, also a lead author, agrees: “Because we had such different areas of expertise, we often had to explain our experiments and analyses to each other. Heart regeneration is a complex process that’s influenced by many different things. The experiments produced enormous quantities of data. Filtering the correct biological signals out of them was hugely challenging.”
It is still unclear whether damaged hearts in mammals like humans and mice lack the necessary signals or the ability to read the signals. If the signals are lacking, medication could eventually be developed to simulate them. But, says Junker, finding a way to mimic signal interpretation would be a great deal more difficult.
Fibroblasts also help to form new blood vessels
The researchers now want to look more closely at the genes that the temporarily activated fibroblasts read especially often. They know that many of the genes in question are important for releasing proteins into the surrounding area. And these might include factors that also influence cardiomyocytes. And initial evidence suggests that the activated fibroblasts don’t just promote the regeneration of the heart; they also help to form new blood vessels that supply the heart with oxygen.
Reference: “Origin and function of activated fibroblast states during zebrafish heart regeneration” by Bo Hu, Sara Lelek, Bastiaan Spanjaard, Hadil El-Sammak, Mariana Guedes Simões, Janita Mintcheva, Hananeh Aliee, Ronny Schäfer, Alexander M. Meyer, Fabian Theis, Didier Y. R. Stainier, Daniela Panáková, and Jan Philipp Junker, 21 July 2022, Nature Genetics.
DOI: 10.1038/s41588-022-01129-5




Be the first to comment on "How a Certain Animal Can Regenerate a Broken Heart"