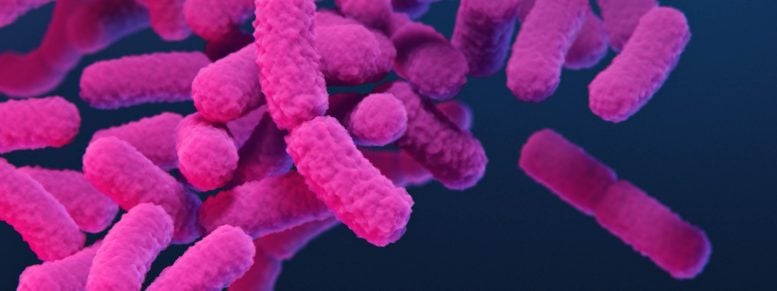
An artist’s rendering of bacteria from the Enterobacter genus. Credit: Center for Disease Control and Prevention
A mechanism used by bacteria to defend themselves could lead to the development of new antibiotics.
Princeton Engineering researchers have found a compound that can kill bacteria that cause incurable infections, with the potential to address the current drug-resistance crisis.
The compound, called cloacaenodin (chloa-say-nodin), is a short, slip-knotted chain of amino acids known as a lasso peptide, encoded by gut-dwelling bacteria as a defense mechanism. Peptides do all kinds of things in the body and have been used in a wide range of medical treatments. This peptide works by attacking rival bacteria, and it’s a very potent killer, according to A. James Link, professor of chemical and biological engineering. If harnessed by science, it could be redirected to fight infections that are not treatable by today’s medicines.
When released, the peptide hooks into a target cell’s RNA-producing enzymes and shuts down basic cell functions. It targets an especially fearsome group of pathogens belonging to the genus Enterobacter, which the Center for Disease Control and Prevention (CDC) has identified as a primary driver in an accelerating global crisis: bacterial infections that increasingly do not respond to conventional antibiotics.
“Not only does [this peptide] kill off-the-shelf, historical Enterobacter strains, it also kills Enterobacter strains that actually have come from patients in the hospital and that are drug-resistant,” said Link, who published a paper on the findings in ACS Infectious Diseases.
Link’s research group has discovered several peptides in this same class — structured with a ring knotted to a tail that threads back down through the ring, like a lasso in a rodeo trick — that show promising antibacterial properties. He said cloacaenodin is unique because it can kill clinically relevant drug-resistant strains, making it a promising subject for antibiotic development. The finding also suggests his peptide-mining and synthetic biology techniques could reveal more antimicrobial compounds with strong drug-development potential, an essential step in quelling the growing superbug crisis.
“If it’s made by one Enterobacter species, it’s likely going to kill other species of Enterobacter. So it’s this sort of guilt-by-association approach,” Link said. This gives researchers a way to prioritize peptide-mining hits since peptides that are encoded in strains related to pathogens are more likely to have interesting bioactivity, he said.
An urgent need for new approaches
Ever since Anne Miller’s fever broke on March 14, 1942, making her the first person ever saved by an antibiotic, humans have been simultaneously staving off deadly bacteria in the short run and saving millions of lives but also making infections harder to treat in the long run. Call it the law of unintended consequences. Some microbes have evolved rapidly to overwhelm our best efforts to destroy them.
The CDC has identified some Enterobacter species as a particularly urgent threat. Although harmless in the human gut, where they are common, when these bacteria enter the airways or urinary tract, they can cause serious infections. Many evade all known medicines, including a highly effective class of antibiotics known as carbapenems. So-called multi-drug resistance has ballooned over the past two decades. Untreatable infections now claim around a million lives each year, with that number projected to surpass cancer’s death toll and reach 10 million per year by 2050, according to a 2019 United Nations report.
Market forces exacerbate the problem, according to the World Health Organization (WHO). Big pharmaceutical companies have strong financial incentives to pursue treatments for chronic conditions, where patient demand stretches over years. Because infections are treated in short finite intervals, profits from new antibiotics are relatively constrained. Adding to that, to slow drug-resistance dynamics, doctors tend to use newer drugs only after older drugs fail, leading to sluggish demand for small firms. And many new antibiotics don’t present a clear advantage over cheaper, more familiar drugs. Over the past decade, several high-profile biotech startup companies with FDA-approved antibiotic treatments have collapsed under these economic conditions.
All of this has slowed the antibiotic-development pipeline to a trickle. The WHO has called the outlook “bleak.” A recent report said that the “lack of diverse compounds suitable for bacterial treatment” and the “absence of new, suitable chemical matter to serve as leads for drug discovery is a major bottleneck in antibiotic discovery.”
The non-profit organization CARB-X, run out of Boston University, has said developing new classes of antibiotics is the best strategy in addressing this urgent need. “You need a diversity of products,” said CARB-X research and development chief Dr. Erin Duffy. “You need antibiotics — things that kill bacteria once you have an infection — and you need different classes, multiple classes.” More than 20 classes of antibiotics were marketed in the two decades after Anne Miller’s miraculous recovery. But since 1962 only two new antibiotic classes have made it to market, and neither treats the most resistant kinds of infections.
“It’s one thing to kill bacteria,” said Drew Carson, a fourth-year Ph.D. student in chemical and biological engineering and the paper’s first author. “It’s another thing to kill bacteria that can actually make people really sick.”
A guilt-by-association approach
While cloacaenodin shows strong antibacterial properties, it’s only the first of many steps to a new treatment. Determining a compound’s safety is difficult and expensive, and moving from initial testing through the regulatory process takes a minimum of 10 years. Duffy said that, historically, some peptides have proven toxic to the kidneys, curbing their use in drugs. But peptides with bacterial-selective activity that don’t harm animal cells will likely lack this toxicity, according to Link.
But this new compound shows promising antibacterial properties and the researchers have only just begun to consider what comes next. They plan to start by testing it in animal infection models to confirm that it can clear the infection and that it is safe for animal cells. More broadly, however, this compound’s discovery suggests that Link and his team have developed a peptide-mining toolkit that will turn up many other interesting compounds in the future, and there is no telling where that will lead.
“The way that we find these peptides is by looking at the genome sequence of an organism,” Link said. “If you give us any DNA sequence, we can very rapidly and very accurately figure out if there’s a lasso peptide encoded within it. We also know about certain sequences within lasso peptides which means there’s a good chance that they’re antimicrobial. And that’s how we homed in on this one.”
Link said there are thousands of Enterobacter genome sequences that have been entered into scientific databases, and the lasso peptide his team discovered is found in only a handful. One of those organisms came from a hospital patient who had a lung infection. And because of his guilt-by-association approach to finding the peptide, they knew it would likely kill many related organisms that don’t have the exact same genes.
“We tested it against a dozen or so strains and saw activity,” Link said, referring to antibacterial activity. “But it potentially has activity against several hundred and maybe even thousands of these sequenced isolates of Enterobacter.”
Reference: “Cloacaenodin, an Antimicrobial Lasso Peptide with Activity against Enterobacter” by Drew V. Carson, Monica Patiño, Hader E. Elashal, Alexis Jaramillo Cartagena, Yi Zhang, Megan E. Whitley, Larry So, Angelo K. Kayser-Browne, Ashlee M. Earl, Roby P. Bhattacharyya and A. James Link, 15 December 2022, ACS Infectious Diseases.
DOI: 10.1021/acsinfecdis.2c00446
The study was funded by the National Institutes of Health.





Bacteria 🦠 is just gross 🤢, it doesn’t matter how we choose to view it, exposure has to exit an immediately. Think about ” sexual” an other body opening holes, for bacteria 🦠🧫 to rest an grow an harbor, an out of control eventually.