Mitochondrial defects caused by rare genetic mutations cause human cells to increase their metabolism. Though that helps short-term survival, it comes at a high cost: a dramatic increase in the rate at which the cells age. Hypermetabolism also may be a key reason why most cells deteriorate as everyone gets older.
The solution could be linked to mitochondria, the energy-providing organelles in cells. This concept is not novel, but until now, there was a lack of direct evidence in human cells.
A recent study published in Communications Biology and led by researchers from Columbia University has uncovered that human cells with impaired mitochondria respond by going into overdrive and using more energy. This process, known as hypermetabolism, allows the cells to temporarily survive, but it also accelerates their aging rate significantly.
“The findings were made in cells from patients with rare mitochondrial diseases, yet they may also have relevance for other conditions that affect mitochondria, including neurodegenerative diseases, inflammatory conditions, and infections,” says principal investigator Martin Picard, Ph.D., associate professor of behavioral medicine (in psychiatry and neurology) at Columbia University Vagelos College of Physicians and Surgeons.
“In addition, hypermetabolism may be a key reason why most cells deteriorate as we get older.”
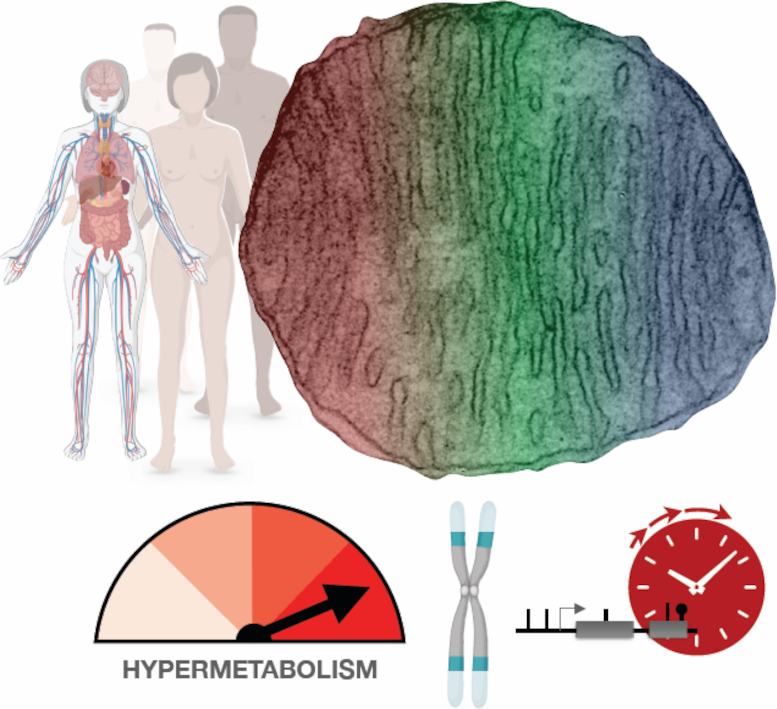
Mitochondrial defects caused by rare genetic mutations cause human cells to increase their metabolism. Though that helps short-term survival, it comes at a high cost: a dramatic increase in the rate at which the cells age. Hypermetabolism also may be a key reason why most cells deteriorate as everyone gets older. Credit: Columbia University Irving Medical Center (Martin Picard)
Hypermetabolic cells age faster
It was generally assumed that mitochondrial defects (which impair the conversion of food sources into usable energy) would force cells to slow their metabolic rate in an effort to conserve energy. However, by analyzing metabolic activity and energy consumption in cells from patients with mitochondrial diseases, the researchers found that cells with impaired mitochondria double their energy expenditure. Moreover, re-analyzing data from hundreds of patients with different mitochondrial diseases showed that mitochondrial defects also increase the energetic cost of living at the whole-body level.
Although this energy boost keeps cells running, it also degrades the cell’s telomeres (caps that protect the ends of our chromosomes) and activates stress responses and inflammation. The net effect accelerates biological aging.
“When cells expend more energy to make proteins and other substances essential for short-term survival, they’re likely stealing resources from processes that ensure long-term survival, like maintaining telomeres,” says Gabriel Sturm, a graduate student and lead author on this study.
Hypermetabolism, fatigue, and aging
This hypermetabolic state could explain why people with mitochondrial diseases experience fatigue and exercise intolerance, among other symptoms. “To make up for the extra energy use in your cells, your body ‘tells’ you not to overexert yourself, to conserve energy. We likely see the same dynamic as people age and their vitality diminishes,” Picard says.
The study doesn’t point to any new remedies for patients with mitochondrial diseases, which are currently not treatable, but it does reinforce the current recommendations for patients to move more. “That may seem counterintuitive since if you’re more active, you’re going to expend more energy and possibly make your symptoms worse,” Sturm says. “But exercise is known to increase the efficiency of an organism. An individual who runs, for example, uses less energy to sustain basic bodily processes than someone who is not physically active.”
Improving organismal efficiency, which would lower energy use in the cells and improve fatigue and other symptoms, may partially explain the health benefits of exercise in patients with mitochondrial diseases and otherwise healthy people.
In their search for new treatments for mitochondrial diseases, researchers should focus on hypermetabolism, Picard says. “Although mitochondrial defects do impair the ability of cells to produce energy, energy deficiency may not be the primary disease initiator. Our study shows these defects increase energy consumption. To move the needle therapeutically, we may need to target hypermetabolism. We need more research to know if that would work.”
Hypermetabolism is also common to other diseases. If increased cellular energy expenditure plays a causal role in driving the aging process, targeting hypermetabolism may be a way to improve fatigue, improve people’s quality of life, or even to slow biological aging.
Reference: “OxPhos defects cause hypermetabolism and reduce lifespan in cells and in patients with mitochondrial diseases” by Gabriel Sturm, Kalpita R. Karan, Anna S. Monzel, Balaji Santhanam, Tanja Taivassalo, Céline Bris, Sarah A. Ware, Marissa Cross, Atif Towheed, Albert Higgins-Chen, Meagan J. McManus, Andres Cardenas, Jue Lin, Elissa S. Epel, Shamima Rahman, John Vissing, Bruno Grassi, Morgan Levine, Steve Horvath, Ronald G. Haller, Guy Lenaers, Douglas C. Wallace, Marie-Pierre St-Onge, Saeed Tavazoie, Vincent Procaccio, Brett A. Kaufman, Erin L. Seifert, Michio Hirano and Martin Picard, 12 January 2023, Communications Biology.
DOI: 10.1038/s42003-022-04303-x
The study was funded by the National Institutes of Health, Baszucki Brain Research Fund, J. Willard and Alice S. Marriott Foundation, Muscular Dystrophy Association, Nicholas Nunno Foundation, JDF Fund for Mitochondrial Research, and the Shuman Mitochondrial Disease Fund.




Amazing website