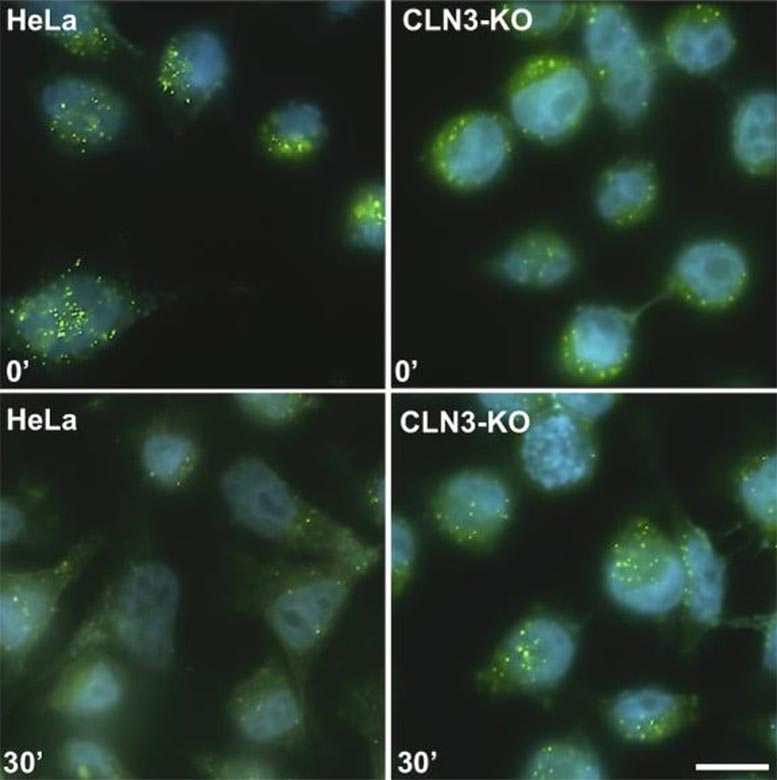
Lysosomal degradation efficiency after 30 minutes. For cells with normal CLN3 protein (left), the green markers are degraded, and when the protein is absent (right), degradation is less efficient. Credit: Stéphane Lefrançois, INRS
Canadian scientists take one step closer to a better understanding of Batten disease.
Professor Stéphane Lefrançois, a researcher at the Institut National de la Recherche Scientifique (INRS), is working on Batten disease, a neurodegenerative genetic disease that primarily affects children. His research focuses on the most common form of the disease — Batten CLN 3 — which is caused by mutations in the protein of the same name and for which there is still no cure.
Children affected by Batten disease are born with no symptoms and develop normally, learning to walk, talk, and interact with others. Between 5 and 8 years of age, however, they start to regress. “The first symptom that leads parents to seek medical attention for their child is a loss of vision caused by retinal degeneration. This is followed by cognitive regression characterized by speech and mobility impairment. The life expectancy for people with the disease is usually around 30 years,” explains Lefrançois, who has been working on Batten disease for more than ten years.
A key protein
Professor Lefrançois and his team in Laval are delving into the cellular biology of the CLN3 protein, which has been synthesized with the help of its namesake gene, in order to better understand the protein’s function and identify therapeutic targets. They recently published findings about a key role played by CLN3 in the Journal of Cell Science. In the absence of the disease, CLN3 ensures a constant supply of proteins to the endosome, an intracellular compartment that serves as a sorting center for proteins within the cell.

Professor Stéphane Lefrançois, a researcher at the Institut National de la Recherche Scientifique (INRS), is working on Batten disease, a neurodegenerative genetic disease that primarily affects children. His research focuses on the most common form of the disease — Batten CLN 3 — which is caused by mutations in the protein of the same name and for which there is still no cure. Credit: INRS
“Under this cellular process, a receptor acts as a truck that carries proteins from the Golgi apparatus, the production factory, to the sorting centre. Thanks to CLN3, this truck normally returns to the Golgi to pick up another load of proteins in an ongoing cycle,” the researcher explains. “In the presence of the mutations, however, the truck doesn’t make the return trip. Instead, it is redirected to the lysosomes, where it’s broken down as cellular waste.”
Because the receptor is degraded, the proteins vital to lysosome function can’t reach their destination. In consequence, these organelles are no longer able to break down cellular waste, so they accumulate and cause cellular degeneration. “We think that children with the disease develop normally in their early years because their cells compensate by making more trucks. It’s possible that the cells can’t keep up, so the system becomes dysfunctional and starts to degrade,” adds Professor Lefrançois.
Professor Lefrançois is working with a team of European researchers to re-establish normal CLN3 function with a promising drug. The aim is to prevent degradation of the receptor so it can continue carrying proteins.
Worldwide, it is estimated that one person in 100,000 has Batten’s disease in all its forms.
About the study
Reference: “CLN3 regulates endosomal function by modulating Rab7A effector interactions” by Seda Yasa, Graziana Modica, Etienne Sauvageau, Abuzar Kaleem, Guido Hermey and Stephane Lefrancois, 7 February 2020, Journal of Cell Science.
DOI: 10.1242/jcs.234047
The article entitled CLN3 regulates endosomal function by modulating Rab7A effector interactions, by Seda Yasa, Graziana Modica, Etienne Sauvageau, Abuzar Kaleem, Guido Hermey, Stephane Lefrancois, was published in the Journal of Cell Science. The research was supported by the Joint Programme on Neurodegenerative Diseases Grant, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, the National Contest for Life Foundation Germany and by the Deutsche Forschungsgemeinschaft, the Fondation Armand-Frappier et du Fonds de recherche du Québec – Santé (FRQS).






Be the first to comment on "Key Role of a Protein Revealed in Rare Disease in Children That Has No Cure"