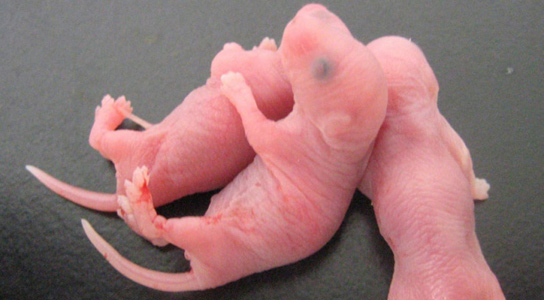
Baby mice born with viable sperm created from stem cells of mice.
Japanese scientists have been able to coax mouse stem cells into becoming viable eggs that were able to produce healthy offspring. This will provide a powerful tool to study the basic elements of mammalian development and infertility.
The researchers published their findings in the journal Science. Scientists have been trying to make sex cells from embryonic stem cells and pluripotent cells for years. Stem-cell scientists have derived many types of cells from stem-cell precursors, but have struggled with sex cells, which have more complex developmental programs because of the way that they divide. Most cells in the body undergo mitosis, in which both sets of chromosomes are copied, but sex cells are produced by meiosis, which results in cells containing only a single copy of each chromosome.

Mouse pups created using lab-made eggs went on to be fully fertile themselves.
Last year, the team from Mitinori Saitou’s lab at Kyoto University in Japan, was able to use mouse stem cells to make sperm. Sperm cells are some of the simpler cells in the body, whereas oocytes are much more complex.
Saitou and his colleagues started with two cell types, mouse embryonic stem cells and induced pluripotent stem cells, which can be derived from adult cells. They used a mixture of signaling molecules to transform the stem cells first into epiblast cells and then into primordial germ cells (PGCs), which are both egg precursors. Male PGCs could be directly injected into infertile mice to mature into sperm; the female version required more coaxing.
The scientists then isolated embryonic ovary tissue that did not contain sex cells and added their own PGCs to the dish. The mixture spontaneously formed ovary-like structures, which were eventually transplanted into female mice. After four weeks, the PGCs had matured into oocytes. They were fertilized and transplanted into foster mothers and the produced offspring grew up to be fertile themselves.
PGCs are scarce and difficult to isolate in mice, so researchers know little about their regulation, states Saitou. As PGCs develop into sperm or egg cells, certain genes are silenced in a process called genomic imprinting. Little is known about how it starts or how genes are selected for silencing.
The exact details of meiosis are also poorly understood, especially in oocytes. Oocytes remain dormant from the point they are formed in the female embryo until the host organism starts ovulating.
The group is now trying to make PGCs from human cells, which could prove to be difficult because of the difference between human and mice stem cells. There are also practical and ethical implications that will limit the researchers’ ability to obtain human ovary tissue.
Reference: “Offspring from Oocytes Derived from in Vitro Primordial Germ Cell–like Cells in Mice” by Katsuhiko Hayashi, Sugako Ogushi, Kazuki Kurimoto, So Shimamoto, Hiroshi Ohta and Mitinori Saitou, 4 October 2012, Science.
DOI: 10.1126/science.1226889







Be the first to comment on "Lab-Made Mouse Oocytes Produce Fertile and Viable Offspring"