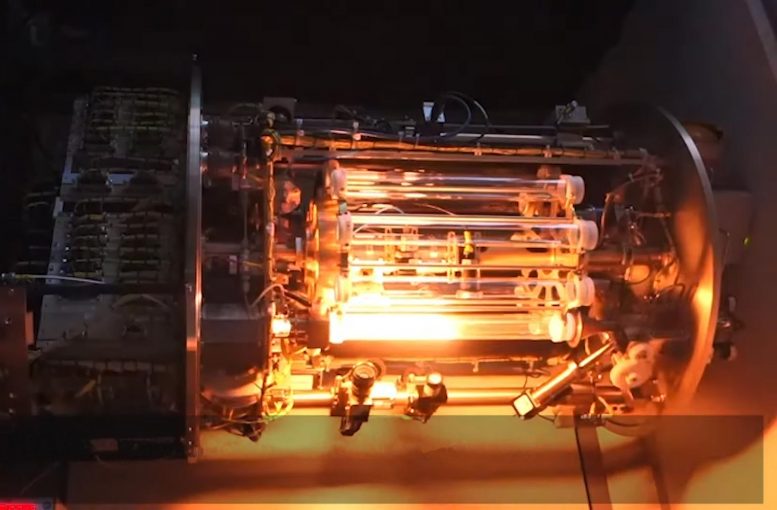
Understanding the combustion physics of metal fuels is crucial for efficient combustion systems. Microgravity is aiding in refining processes for burning solid fuels like iron dust.
Did you know that in microgravity we are preparing one of the most promising fuels for the future?
Microgravity is helping to find answers and models to refine the processes needed to efficiently burn solid fuel like iron dust. Are we witnessing the rise of a new “Iron Age”? Could we use metal powders instead of petrol to fuel our cars?
Solid fuels are used for burning a match, lighting a sparkler on New Year’s Eve as well as the fuel inside the boosters of Ariane and of other rockets. But metals such as iron can also burn, in powder form, and are entirely smokeless and carbon free.
Metals could be produced using clean energy, such as from solar cells or wind turbines. That electricity is stored as chemical energy in the metal powder at energy densities that are competitive with fossil fuels. This has the potential to reduce greenhouse gas emissions globally, but a barrier to implementing this technology is the development of combustion systems that can efficiently burn the metal fuels, which requires a solid understanding of their combustion physics.
To understand the physics of metal fuel combustion, a cluster of iron powder needs to be suspended for about 30 seconds, the time needed to observe and study how a flame propagates. Researchers used sounding rockets and parabolic flights to run experiments in weightlessness and to validate existing models, yielding promising results.
The density of iron particles and the composition of gases in the combustion chamber are essential parameters, like in a petrol car engine. Microgravity allows for the study of the laws of flame propagation, to optimize parameters in industrial burner designs, and reduce impact on the environment.
These space experiments also help us understand similar phenomena, such as the spreading of contagious microbes and forest fires.
In a vote of confidence for the technique, a student team at TU Eindhoven in The Netherlands worked with industrial partners to design a metal combustion facility now installed at Swinkels Family Brewers, subsidized by the Dutch province of Noord-Brabant, used to produce steam for the brewing process.








A major use of fossil fuels (FF) is to power vehicles. Liquid FF have the advantage of high energy density, easy storage, and easy transport to the engine. There are reasons that we don’t use coal dust in cars and trucks. The reasons are the same for powdered metals. Powders are not as easy to transport, store, and move to the engine. However, there is a much more important consideration. What to do with the iron oxide (rust) that results from the ‘combustion?’ You can’t just dump it on the highways. We’d be plowing our way through orange ‘snow.’ It you capture it and keep it onboard, it increases the weight of the vehicle and reduces the efficiency of the fuel. What do you do with it when you get home? You can’t dump it in your yard. In fact, it you live in a humid climate, it may almost be impossible to get it out of the storage compartment. Even if you do, then what do you do with it? It becomes yet another waste product to handle. If there is a loss of the iron fuel or iron oxide waste-product to the environment, it could become a serious hazard to breathing, and even to plants, depending on the particle size. Burning iron may find niche applications, but I don’t see it replacing conventional liquid fuels.
I assume they are considering this for use in static applications rather than for transport where the weight would be prohibitive for the performance and competing with electric vehicles almost impossible.
If this can be accomplished efficiently at scale then it could be a useful means of energy storage for electricity generation, but right now that seems very far off.
During temperature changes, water condenses in my car’s gas tank. I wonder what real world conditions will do for this fuel.
“which requires a solid understanding of their combustion physics” 😉
Yeah, no carbon? Anything that burns uses an oxygen and produces carbon. https://www.google.com/url?sa=t&source=web&rct=j&url=https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Electrochemistry/Redox_Chemistry/Definitions_of_Oxidation_and_Reduction&ved=2ahUKEwiyo_mWlKbuAhWrtVkKHYzFAPMQFjAJegQIFhAJ&usg=AOvVaw3qJuzR2Vz2yTnaZPvGRmqo
Someone who apparently has never witnessed magnesium burning in a basic chemistry class shouldn’t try to read journals…