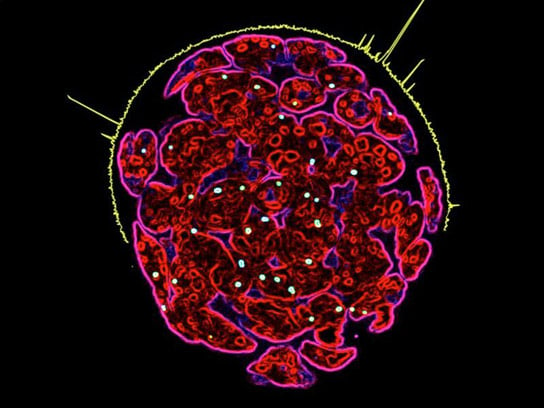
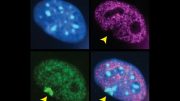
Immunofluorescence image of a multicellular colony of female mouse embryonic stem cells: in culture, the cells were probed with RNA-FISH probe specific for Tsix-DXPas34 (green/yellow dots). The yellow signal surrounding the upper hemisphere of the cell colony is the ChIP-Sequencing readout for MSL2 chromatin binding in the region of the X inactivation center. The most pronounced peak showcases the binding of MSL2 to Tsix enhancer – DXPas34. Credit: MPI f. Immunobiology and Epigenetics/ Tomasz Chelmicki
Scientists from the Max Planck Institute have discovered that the same evolutionary stable MOF protein that regulates X dosis in flies is also involved in compensatory mechanisms in mice.
Sexually dimorphic animals are often distinguished by unequal numbers of X chromosomes. While males have only one X chromosome, females have two copies, prompting an evolutionary pressure for compensatory mechanisms against this disequilibrium. Some species, such as fruit flies, up-regulate the single X chromosome in males, while other species, such as mouse and human, silence one of the two X in females. Now scientists from the Max Planck Institute of Immunobiology and Epigenetics in Freiburg have discovered that the same, evolutionary stable MOF protein that regulates X dosis in flies is also involved in compensatory mechanisms in mice. Interestingly, MOF-mediated regulation of X-inactivation is achieved by parallel action of not one, but two distinct complexes.
In male fruit flies the protein complex called MSL together with its major enzyme called MOF pushes the single X chromosome to express its genes with double efficiency. Mice also struggle with different numbers of X’s between the sexes, but in contrast to flies, where ‘X up-regulation’ takes place, here the females inactivate one of their two X chromosomes in a process called ‘X inactivation’.
The team, led by Asifa Akhtar, Director at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg demonstrated that two – throughout the evolution extremely conserved – protein complexes have an impact on upregulation and inactivation. Both complexes orchestrate the function of the gene regulator MOF. “What we find most intriguing is: MOF and its protein partners maintain the activity of both X chromosomes in female stem cells, which is essential for preserving their unique character,” said Akhtar. “It was overwhelming for us to see that the same protein engages in the specific regulation of X chromosomal dose both in flies as well as mouse, where these mechanisms seem world apart,” continued the co-lead author Tomasz Chelmicki. In addition, the MOF-associated complexes influence the expression of thousands of genes in mouse cells.
During development of female mammals one of the two X chromosomes has to be inactivated in order to achieve the identical number of genes in male and female individuals, a process called ‘dosage compensation’. However, in embryonic stem cells both X chromosomes have to remain active. The study now shows that the MOF protein complex plays a central role in this X chromosome regulation. The MOF-MSL complex regulates the gene Tsix, which inhibits the production of Xist – an RNA molecule responsible for X chromosome inactivation. The protein complex MOF-NSL ensures the preservation of stem cell identity through activation of several factors and thus effectively antagonizing the expression of the RNA Xist, that would ultimately lead to X-inactivation.
Detailed insights into genome-wide interactions of MSL and NSL were possible due to a combination of powerful sequencing techniques and biochemical experiments. “Analyzing the continuously growing amount of data that we obtained with high-throughput methods is a real challenge,” said co-lead author Friederike Dündar. “But it also offers the opportunity to study how different complexes can cooperate and complement each other in order to reach the same goal in the cell.”
The MOF enzyme is responsible for histone acetylation. This posttranslational modification results in a better accessibility of the expression machinery to the DNA. The knowledge gained from this study will pave the way towards a better understanding of complex processes such as embryonic development, organogenesis, and pathogenesis of disorders like cancer.
Reference: “MOF-associated complexes ensure stem cell identity and Xist repression” by Tomasz Chelmicki, Friederike Dündar, Matthew James Turley, Tasneem Khanam, Tugce Aktas, Fidel Ramírez, Anne-Valerie Gendrel, Patrick Rudolf Wright, Pavankumar Videm, Rolf Backofen, Edith Heard, Thomas Manke and Asifa Akhtar, 19 May 2014, eLife.
DOI: 10.7554/eLife.02024




X- chromosome inactivation is common in embryo, where the inactivated X-chromosome becomes a barr body. Sometimes inactivated X chromosome is not fully silenced and it may be the reason for LGBT behavior. In animals and some aquatic species, the inactivation leads to Male or Female offspring which is purely based on environment. Thank YOu.