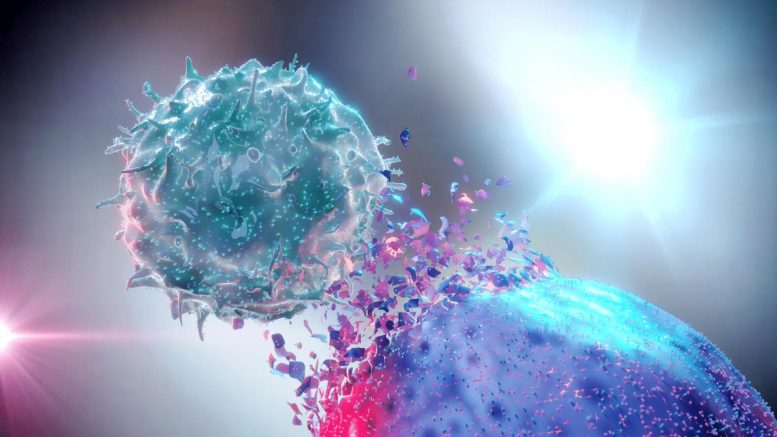
The novel approach combines traditional chemotherapy drugs with a new method of tumor irradiation.
A combination of internal radiation and chemotherapy dissolves tumors in 80% of mice across multiple models.
Duke University biomedical engineers have demonstrated the most effective pancreatic cancer treatment yet recorded in mouse models. While most mouse trials consider just stopping growth to be a success, the new treatment fully eliminated tumors in 80% of mice across many model types, including those considered to be the most difficult to treat.
The approach combines traditional chemotherapy drugs with a new method for irradiating the tumor. The treatment implants radioactive iodine-131 directly into the tumor inside a gel-like depot that protects healthy tissue and is absorbed by the body once the radiation fades, as opposed to administering radiation from an external beam that passes through healthy tissue.
The study was recently published in the journal Nature Biomedical Engineering.
“We did a deep dive through over 1100 treatments across preclinical models and never found results where the tumors shrank away and disappeared like ours did,” said Jeff Schaal, who conducted the research during his Ph.D. in the laboratory of Ashutosh Chilkoti, the Alan L. Kaganov Distinguished Professor of Biomedical Engineering at Duke. “When the rest of the literature is saying that what we’re seeing doesn’t happen, that’s when we knew we had something extremely interesting.”
Pancreatic cancer is the third leading cause of cancer-related mortality but accounts for just 3.2% of all cancer cases. It is incredibly difficult to treat since its tumors tend to have aggressive genetic mutations that make it resistant to many drugs, and it is usually identified very late, once it has spread to other parts of the body.
The current leading treatment combines chemotherapy, which keeps cells in a reproductive stage vulnerable to radiation for prolonged periods of time, with a radiation beam directed at the tumor. However, this method is ineffective until a certain level of radiation reaches the tumor. Moreover, despite recent breakthroughs in radiation beam shaping and targeting, reaching that threshold without risking severe side effects is very challenging.
Another method researchers have tried involves implanting a radioactive sample encased in titanium directly within the tumor. But because titanium blocks all radiation other than gamma rays, which travel far outside the tumor, it can only remain within the body for a short period of time before damage to surrounding tissue begins to defeat the purpose.
“There’s just no good way to treat pancreatic cancer right now,” said Schaal, who is now director of research at Cereius, Inc., a Durham, North Carolina biotechnology startup working to commercialize a targeted radionuclide therapy through a different technology scheme.
To skirt these issues, Schaal decided to try a similar implantation method using a substance made of elastin-like polypeptides (ELPs), which are synthetic chains of amino acids bonded together to form a gel-like substance with tailored properties. Because ELPs are a focus of the Chilkoti lab, he was able to work with colleagues to design a delivery system well-suited for the task.
The ELPs exist in a liquid state at room temperature but form a stable gel-like substance within the warmer human body. When injected into a tumor along with a radioactive element, the ELPs form a small depot encasing radioactive atoms. In this case, the researchers decided to use iodine-131, a radioactive isotope of iodine, because doctors have used it widely in medical treatments for decades and its biological effects are well understood.
The ELP depot encases the iodine-131 and prevents it from leaking out into the body. The iodine-131 emits beta radiation, which penetrates the bio gel and deposits almost all its energy into the tumor without reaching the surrounding tissue. Over time, the ELP depot degrades into its constituent amino acids and is absorbed by the body — but not before the iodine-131 has decayed into a harmless form of xenon.
“The beta radiation also improves the stability of the ELP bio gel,” Schaal said. “That helps the depot last longer and only break down after the radiation is spent.”
In the new paper, Schaal and his collaborators in the Chilkoti laboratory tested the new treatment in concert with paclitaxel, a commonly used chemotherapy drug, to treat various mouse models of pancreatic cancer. They chose pancreatic cancer because of its infamy for being difficult to treat, hoping to show that their radioactive tumor implant creates synergistic effects with chemotherapy that relatively short-lived radiation beam therapy does not.
The researchers tested their approach on mice with cancers just under their skin created by several different mutations known to occur in pancreatic cancer. They also tested it on mice that had tumors within the pancreas, which is much more difficult to treat.
Overall, the tests saw a 100% response rate across all models, with the tumors being completely eliminated in three-quarters of the models about 80% of the time. The tests also revealed no immediately obvious side effects beyond what is caused by chemotherapy alone.
“We think the constant radiation allows the drugs to interact with its effects more strongly than external beam therapy allows,” Schaal said. “That makes us think that this approach might actually work better than external beam therapy for many other cancers, too.”
The approach, however, is still in its early preclinical stages and will not be available for human use anytime soon. The researchers say their next step is large animal trials, where they will need to show that the technique can be accurately done with the existing clinical tools and endoscopy techniques that doctors are already trained on. If successful, they look toward a Phase 1 clinical trial in humans.
“My lab has been working on developing new cancer treatments for close to 20 years, and this work is perhaps the most exciting we have done in terms of its potential impact, as late-stage pancreatic cancer is impossible to treat and is invariably fatal,” Chilkoti said. “Pancreatic cancer patients deserve better treatment options than are currently available, and I am deeply committed to taking this all the way into the clinic.”
Reference: “Brachytherapy via a depot of biopolymer-bound 131I synergizes with nanoparticle paclitaxel in therapy-resistant pancreatic tumours” by Jeffrey L. Schaal, Jayanta Bhattacharyya, Jeremy Brownstein, Kyle C. Strickland, Garrett Kelly, Soumen Saha, Joshua Milligan, Samagya Banskota, Xinghai Li, Wenge Liu, David G. Kirsch, Michael R. Zalutsky, and Ashutosh Chilkoti, 19 October 2022, Nature Biomedical Engineering.
DOI: 10.1038/s41551-022-00949-4
The study was funded by the National Institutes of Health and the National Cancer Institute.




Pancreatic cancer is horrible. Hard to detect early and hard to treat. Any good treatment is fantastic.
I repeat, this is great stuff until Big Pharma waves a big fat check in front of the researchers’ eyes and takes this discovery, and tosses it. Big Pharma wants nothing to do with cancer “cures” because they make too much money pushing their failed “chemo” bullcrap. Always remember folks, it’s more profitable to “treat” than to CURE.
This type of treatment has been around for years. It’s called Isopet from Vivos. The company is working towards FDA approval for humans.
I saw the blood clot removal wire that extends & retracts. How about a long those lines for administering the receiving part
I saw this NBC news article in June of this year reporting the success of a new type of T-cell therapy which had remarkable effect for a woman with stage 4 pancreatic cancer. https://www.nbcnews.com/health/health-news/gene-therapy-pancreatic-cancer-experimental-approach-shrank-tumors-one-rcna31437
The researchers said it has great potential for many other cancer types.
Keep at it,the more clinical reaserch energy will produce something positive at some point.
Not to be a debbie downer, but most pancreatic cancer has metastasized by the time it is diagnosed, meaning it has spread elsewhere. Doubtful this will work in those cases because you often don’t know where it has spread. It’s not the pancreatic tumor that kills you but the tumors that spread to the lymph, brain, or lungs.
Use it on dying stage 4 humans instead of large animals which is cruel and in no way the same as in people.
Too late for swazy and landon
I’m in recovery now from stage 3 due to an implant of Oncasil ( phosphorous 192 ) all scans show clear for 12 months.