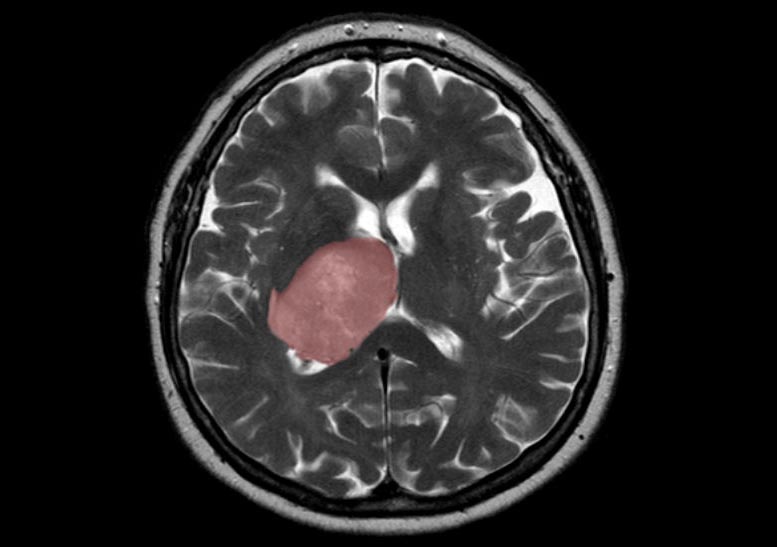
A brain scan showing a top down view of a cross-section with a glioblastoma tumor highlighted in red. Credit: Hellerhoff, Wikimedia Commons
Increase of inflammation markers in tumor-initiating cells believed to be a response to brain injury.
The healing process that follows a brain injury could spur tumor growth when new cells generated to replace those lost to the injury are derailed by mutations, Toronto scientists have found. A brain injury can be anything from trauma to infection or stroke.
The findings were made by an interdisciplinary team of researchers from the University of Toronto, The Hospital for Sick Children (SickKids) and the Princess Margaret Cancer Centre who are also on the pan-Canadian Stand Up To Cancer Canada Dream Team that focuses on a common brain cancer known as glioblastoma.
“Our data suggest that the right mutational change in particular cells in the brain could be modified by injury to give rise to a tumor,” says Dr. Peter Dirks, Dream Team leader who is the Head of the Division of Neurosurgery and a Senior Scientist in the Developmental and Stem Cell Biology program at SickKids.
Gary Bader, a professor of molecular genetics in the Donnelly Centre for Cellular and Biomolecular Research at U of T’s Temerty Faculty of Medicine, and Dr. Trevor Pugh, Senior Scientist at the Princess Margaret, also led the research which has been published today in the journal Nature Cancer.
The findings could lead to new therapy for glioblastoma patients who currently have limited treatment options with an average lifespan of 15 months after diagnosis.
“Glioblastoma can be thought of as a wound that never stops healing,” says Dirks. “We’re excited about what this tells us about how cancer originates and grows and it opens up entirely new ideas about treatment by focusing on the injury and inflammation response.”
The researchers applied the latest single-cell RNA sequencing and machine learning technologies to map the molecular make-up of the glioblastoma stem cells (GSCs), which Dirks’ team previously showed are responsible for tumor initiation and recurrence after treatment.
They found new subpopulations of GSCs that bear the molecular hallmarks of inflammation and which are commingled with other cancer stem cells inside patients’ tumors. It suggests that some glioblastomas start to form when the normal tissue healing process, which generates new cells to replace those lost to injury, gets derailed by mutations, possibly even many years before patients become symptomatic, Dirks said.
Once a mutant cell becomes engaged in wound healing, it cannot stop multiplying because the normal controls are broken and this spurs tumor growth, according to the study.
“The goal is to identify a drug that will kill the glioblastoma stem cells,” says Bader, whose graduate student Owen Whitley contributed to the computational data analysis “But we first needed to understand the molecular nature of these cells in order to be able to target them more effectively.”
The team collected GSCs from 26 patients’ tumors and expanded them in the lab to obtain sufficient numbers of these rare cells for analysis. Almost 70,000 cells were analyzed by single-cell RNA sequencing which detects what genes are switched on in individual cells, an effort led by Laura Richards, a graduate student in Pugh’s lab.
The data confirmed extensive disease heterogeneity, meaning that each tumor contains multiple subpopulations of molecularly distinct cancer stem cells, making recurrence likely as existing therapy can’t wipe out all the different subclones.
A closer look revealed that each tumor has either of the two distinct molecular states — termed “Developmental” and “Injury Response” — or somewhere on a gradient between the two.
The developmental state is a hallmark of the glioblastoma stem cells and resembles that of the rapidly dividing stem cells in the growing brain before birth.
But the second state came as a surprise. The researchers termed it “Injury Response” because it showed an upregulation of immune pathways and inflammation markers, such as interferon and TNFalpha, which are indicative of wound healing processes.
These immune signatures were only picked up thanks to the new single-cell technology after being missed by older methods for bulk cell measurements.
Meanwhile, experiments led by Stephane Angers’ lab at the Leslie Dan Faculty of Pharmacy established that the two states are vulnerable to different types of gene knock outs, revealing a swathe of therapeutic targets linked to inflammation that had not been previously considered for glioblastoma.
Finally, the relative comingling of the two states was found to be patient-specific, meaning that each tumor was biased either toward the developmental or the injury response end of the gradient. The researchers are now looking to target these biases for tailored therapies.
“We’re now looking for drugs that are effective on different points of this gradient”, says Pugh, who is also the Director of Genomics at the Ontario Institute for Cancer Research. “There’s a real opportunity here for precision medicine — to dissect patients’ tumors at the single cell level and design a drug cocktail that can take out more than one cancer stem cell subclone at the same time.”
Reference: “Gradient of Developmental and Injury Response transcriptional states defines functional vulnerabilities underpinning glioblastoma heterogeneity” by Laura M. Richards, Owen K. N. Whitley, Graham MacLeod, Florence M. G. Cavalli, Fiona J. Coutinho, Julia E. Jaramillo, Nataliia Svergun, Mazdak Riverin, Danielle C. Croucher, Michelle Kushida, Kenny Yu, Paul Guilhamon, Naghmeh Rastegar, Moloud Ahmadi, Jasmine K. Bhatti, Danielle A. Bozek, Naijin Li, Lilian Lee, Clare Che, Erika Luis, Nicole I. Park, Zhiyu Xu, Troy Ketela, Richard A. Moore, Marco A. Marra, Julian Spears, Michael D. Cusimano, Sunit Das, Mark Bernstein, Benjamin Haibe-Kains, Mathieu Lupien, H. Artee Luchman, Samuel Weiss, Stephane Angers, Peter B. Dirks, Gary D. Bader and Trevor J. Pugh, 4 January 2021, Nature Cancer.
DOI: 10.1038/s43018-020-00154-9







What? Me Worry?
Wildlife goes extinct at associate degree dreadful rate as a result of busy motorways, categorical ways in which and highways and fences currently separate migratory routes and environments then on prime of that habitat loss and hunters are having an enormous impact on their population numbers. wherever the road are raised each few hundred meters such roads ought to have a a pair of meter diameter concrete pipe tunnel researching where the animals may see the opposite facet to pass through. wherever it’s below ground level, going through cuttings in hills there should be bridges for wild life and other people to pass over.