
Electrocatalyst converts CO2 into multicarbon products.
A new electrocatalyst called a-CuTi@Cu converts carbon dioxide (CO2 ) into liquid fuels. As reported by a team of Chinese researchers in the journal Angewandte Chemie, active copper centered on an amorphous copper/titanium alloy produces ethanol, acetone, and n-butanol with high efficiency.
Most of our global energy demands are still being met by burning fossil fuels, which contributes to the greenhouse effect through the release of CO2. To reduce global warming, we must look for opportunities to use CO2 as a raw material for basic chemicals. Through electrocatalytic conversion of CO2 using renewable energy, a climate-neutral, artificial carbon cycle could be established. Excess energy produced by photovoltaics and wind energy could be stored through the electrocatalytic production of fuels from CO2. These could then be burned as needed. Conversion into liquid fuels would be advantageous because they have high energy density and are safe to store and transport. However, the electrocatalytic formation of products with two or more carbon atoms (C2+) is very challenging.
A team from Foshan University (Foshan, Guangdong), the University of Science and Technology of China (Hefei, Anhui), and Xi’an Shiyou University (Xi’an, Shaanxi), led by Fei Hu, Tingting Kong, Jun Jiang, and Yujie Xiong has now developed a novel electrocatalyst that efficiently converts CO2 to liquid fuels with multiple carbon atoms (C2–4). The primary products are ethanol, acetone, and n-butanol.
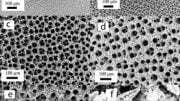
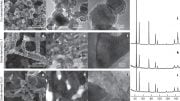
To make the electrocatalyst, thin ribbons of a copper/titanium alloy are etched with hydrofluoric acid to remove the titanium from the surface. This results in a material named a-CuTi@Cu, with a porous copper surface on an amorphous CuTi alloy. It has catalytically active copper centers with remarkably high activity, selectivity, and stability for the reduction of CO2 to C2+ products (total faradaic efficiency of about 49 % at 0.8 V vs. reversible hydrogen electrode for C2–4, and it is stable for at least three months). In contrast, pure copper foil produces C1 products but hardly any C2+ products.
The reaction involves a multistep electron-transfer process via various intermediates. In the new electrocatalyst, the inactive titanium atoms below the surface actually play an important role; they increase the electron density of the Cu atoms on the surface. This stabilizes the adsorption of *CO, the key intermediate in the formation of multicarbon products, allows for high coverage of the surface with *CO, and lowers the energy barrier for di- and trimerization of the *CO as new carbon–carbon bonds are formed.
Reference: “Ultrastable Cu Catalyst for CO2 Electroreduction to Multicarbon Liquid Fuels by Tuning C–C Coupling with CuTi Subsurface” by Prof. Fei Hu, Dr. Li Yang, Yawen Jiang, Dr. Chongxiong Duan, Dr. Xiaonong Wang, Longjiao Zeng, Xuefeng Lv, Delong Duan, Qi Liu, Prof. Tingting Kong, Prof. Jun Jiang, Ran Long and Prof. Yujie Xiong, 1 October 2021, Angewandte Chemie.
DOI: 10.1002/anie.202110303
Dr. Yujie Xiong is the Chair Professor of Chemistry at the University of Science and Technology of China. His main specialty is the artificial carbon cycle.





Amazing! Turning CO2 into fuel kills two birds with one stone. Obviously the question is, at what cost?
The products are all still carbon compounds. Ethanol for biofuels is produced by photosynthesis, bur it is immediately used in transportation. It is 90% fossil fuel and will be required for any of this “chemistry” to take place in the real world.
Possibly a positive step. What is the percentage of greenhouse emissions released from burning ethanol versus gasoline? #1, and #2, engine technology needs to improve in order to efficiently burn higher %’s of ethanol, and possibly retrofit to existing vehicles to become affordable.
#3, what would we do with an exponential increase in acetone? #4, is n-butanol, butane, and could that be used for combustion, what is it’s greenhouse contribution? If not, what would we do with an exponential increase in n-butanol supplies? There is no such thing as a free lunch, everything has a cost.
The answer is 28% less CO2 emissions for ethanol vs. gasoline.
Depending where you live the gasoline composition may vary, but on average one liter of gasoline contains 32 MJ of energy, whereas ethanol contains 22,7 MJ per liter. Less energy means shorter driving range for the car and more frequent gas station stops.
Another important factors to consider are ethanol production and logistics emissions. This applies to those other compounds you mentioned. I will use below scientific units in my calculation below, but convert those to gallons and lbs too.
Gasoline
74 gCO2 per MJ in combustion
14 gCO2 per MJ in prod. and logistics
In total 88 gCO2 per MJ
That is 2816 gCO2 per liter or 4,85 lbs CO2 per gallon
Ethanol
59,6 gCO2 per MJ
4,2 gCO2 per MJ
In total 63,4 gCO per MJ
That is 1448,3 gCO2 per liter or 2,50 lbs per gallon
Next factor in the shorter range: Ethanol 2,50 * 32/22,7=3,50 vs. 4,85 lbs-CO2 per gallon
That is, 28% less CO2 emissions.
You also easily notice how important part of the total emissions is the production and logistics in favor of ethanol. Please familiasize yourself with how the ethanol is produced, where you live. Wheat/grains cause higher emissions than sugarcane in ethanol production and logistics.
Calculation also shows ethanol isn’t a micracle to save our planet. Similar result you can calculate the other chemical compounds you listed.
Last but not least, before you rush to buy a FlexiFuel kit to your car, please check it supports use of ethanol. In many cars, the rubber seals do not withstand ethanol, or the other compounds.
Go to osf on Google look up Theory on a scientific method of atomic co2 conversions leading to gasoline and diesel from vehicle emissions look at time uploaded I published this thesis first china has stolen my reasearch
Where does the hydrogen come from?