Tremendous amounts of hydrogen and methane can be stored in nanoscopic pores.
A research team led by Northwestern University has designed and synthesized new materials with ultrahigh porosity and surface area for the storage of hydrogen and methane for fuel cell-powered vehicles. These gases are attractive clean energy alternatives to carbon dioxide-producing fossil fuels.
The designer materials, a type of a metal-organic framework (MOF), can store significantly more hydrogen and methane than conventional adsorbent materials at much safer pressures and at much lower costs.
“We’ve developed a better onboard storage method for hydrogen and methane gas for next-generation clean energy vehicles,” said Omar K. Farha, who led the research. “To do this, we used chemical principles to design porous materials with precise atomic arrangement, thereby achieving ultrahigh porosity.
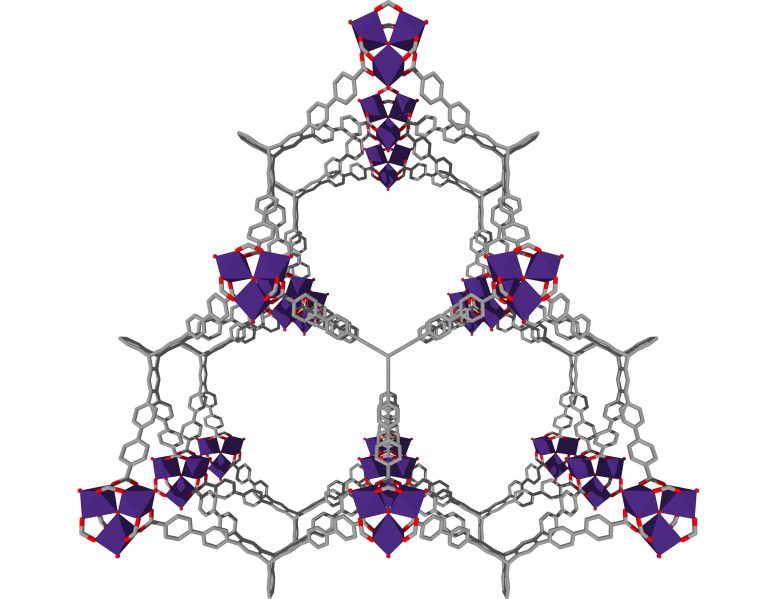
Adsorbents are porous solids which bind liquid or gaseous molecules to their surface. Thanks to its nanoscopic pores, a one-gram sample of the Northwestern material (with a volume of six M&Ms) has a surface area that would cover 1.3 football fields.
The new materials also could be a breakthrough for the gas storage industry at large, Farha said, because many industries and applications require the use of compressed gases such as oxygen, hydrogen, methane, and others.
Farha is an associate professor of chemistry in the Weinberg College of Arts and Sciences. He also is a member of Northwestern’s International Institute for Nanotechnology.
The study, combining experiment and molecular simulation, was published today (April 17, 2020) by the journal Science.
Farha is the lead and corresponding author. Zhijie Chen, a postdoctoral fellow in Farha’s group, is co-first author. Penghao Li, a postdoctoral fellow in the lab of Sir Fraser Stoddart, Board of Trustees Professor of Chemistry at Northwestern, also is a co-first author. Stoddart is an author on the paper.
The ultraporous MOFs, named NU-1501, are built from organic molecules and metal ions or clusters which self-assemble to form multidimensional, highly crystalline, porous frameworks. To picture the structure of a MOF, Farha said, envision a set of Tinkertoys in which the metal ions or clusters are the circular or square nodes and the organic molecules are the rods holding the nodes together.
Hydrogen- and methane-powered vehicles currently require high-pressure compression to operate. The pressure of a hydrogen tank is 300 times greater than the pressure in car tires. Because of hydrogen’s low density, it is expensive to accomplish this pressure, and it also can be unsafe because the gas is highly flammable.
Developing new adsorbent materials that can store hydrogen and methane gas onboard vehicles at much lower pressures can help scientists and engineers reach U.S. Department of Energy targets for developing the next generation of clean energy automobiles.
To meet these goals, both the size and weight of the onboard fuel tank need to be optimized. The highly porous materials in this study balance both the volumetric (size) and gravimetric (mass) deliverable capacities of hydrogen and methane, bringing researchers one step closer to attaining these targets.
“We can store tremendous amounts of hydrogen and methane within the pores of the MOFs and deliver them to the engine of the vehicle at lower pressures than needed for current fuel cell vehicles,” Farha said.
The Northwestern researchers conceived the idea of their MOFs and, in collaboration with computational modelers at the Colorado School of Mines, confirmed that this class of materials is very intriguing. Farha and his team then designed, synthesized and characterized the materials. They also collaborated with scientists at the National Institute for Standards and Technology (NIST) to conduct high-pressure gas sorption experiments.
Reference: “Balancing volumetric and gravimetric uptake in highly porous materials for clean energy” by Zhijie Chen, Penghao Li, Ryther Anderson, Xingjie Wang, Xuan Zhang, Lee Robison, Louis R. Redfern, Shinya Moribe, Timur Islamoglu, Diego A. Gómez-Gualdrón, Taner Yildirim, J. Fraser Stoddart and Omar K. Farha, 17 April 2020, Science.
DOI: 10.1126/science.aaz8881
The research was supported by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy (award no. DE‐EE0008816).







“and it also can be unsafe because the gas is highly flammable.”
It was established long ago that Hydrogen is far more safe that liquid gasoline. Carbon fiber tanks at 10,000 psi can withstand the impact of a .45 pistol shot at close range, or the impact of a semi-truck at highway speeds. If the hydrogen does leak, it rises into the atmosphere immediately and is not a hazard. Gasoline (vapor – the liquid is not flammable) is heavier than air and lingers around the leak sight, and remains a significant fire hazard. The U.S. Army did tests long ago: they did puncture the tank installed in a car and ignited the fuel leak, and a “jet flame” burned through the trunk until the hydrogen was exhausted. The rear glass window of the car remained relatively cool. The passenger compartment was unaffected. Then they did the same thing with a gasoline tank in the same vehicle, and it burned to the ground.
We are being prevented from using any fuel except petroleum-oil based fuels because the world is required to buy and sell oil in U.S. Dollars. This gives the U.S. virtually complete control of the world’s economy, since any country that wants to use oil must allow their banks to interface with U.S. banks; the U.S. can then sanction them for any reason it deams necessary, and the U.S. does impose these restrictions, such as for example (at least in the past) if a country legalized marijuana they could no longer do business with the U.S. in any form.
This is why the U.S. dollar is valued in virtually every country in the world.
At the cost of your children’s health.
Would have liked to see a little quantitative detail. I know its early days in the research but not too early for an estimate of how much hydrogen might be stored in this crystalline material, in the space of (say) a typical gasoline car petrol tank. All we are told is a “tremendous amount”.