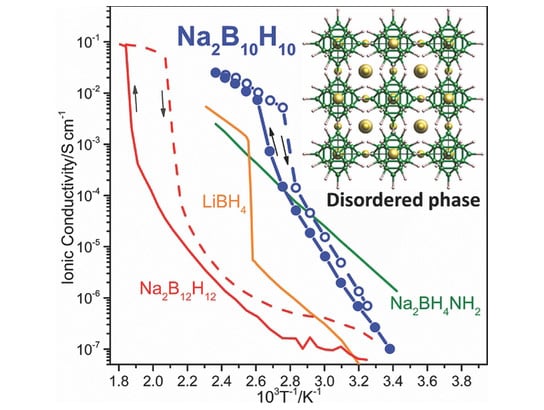
Na2B10H10 exhibits exceptional superionic conductivity above ca. 360 K (e.g., ≈0.01 S cm−1 at 383 K) concomitant with its transition from an ordered monoclinic structure to a face-centered-cubic arrangement of orientationally disordered B10H10 2− anions harboring a vacancy-rich Na+ cation sublattice. This discovery represents a major advancement for solid-state Na+ fast-ion conduction at technologically relevant device temperatures. Credit: Terrence J. Udovic, et al.
Scientists have discovered a sodium-based complex metal hydride material that has the potential to be a much cheaper alternative to the lithium-based conductors used in many rechargeable batteries today.
Rechargeable battery manufacturers may get a jolt from research performed at the National Institute of Standards and Technology (NIST) and several other institutions, where a team of scientists has discovered a safe, inexpensive, sodium-conducting material that significantly outperforms all others in its class.
The team’s discovery is a sodium-based, complex metal hydride, a material with potential as a much cheaper alternative to the lithium-based conductors used in many rechargeable batteries. Because lithium is a comparatively rare commodity near the earth’s surface, the industry would prefer to build reusable batteries out of common ingredients that are both economical and inexhaustible.
The novel hydride—which has the formula Na2B10H10—might fit the bill, and not only because it is formed of the three easily obtainable elements of sodium, boron, and hydrogen. There are other practical reasons as well: It is a stable inorganic solid, meaning it would pose fewer of the risks carried by many flammable liquids in traditional batteries, such as the potential for leaking or exploding. And compared to other sodium-based solids, it can enable more power output.
This last advantage stems from its unusual ability to conduct sodium ions exceptionally well when heated. At room temperature, the hydride’s atoms are tightly packed together. But when heated to near water’s boiling point, they repack to create numerous corridors through which the sodium ions can flow easily. Because charged ions are what carry electricity in a battery, this “phase change,” as physicists call it, allows the team’s material to outperform others.
“It’s more than 20 times better at doing its job than other known sodium-based complex hydrides in this temperature range,” says Terrence Udovic of the NIST Center for Neutron Research (NCNR). “It’s also as good as the best solid lithium-based hydride that has been measured, so it’s quite promising.”
Udovic had been exploring metal hydride materials as candidates for hydrogen storage, and while this particular compound performed poorly at that task, he hit upon the idea of testing it as an ion conductor. NCNR research hinted at its abilities, but clarifying them took an international effort among collaborators from Japan’s Tohoku University, Russia’s Institute of Metal Physics, the University of Maryland, and Sandia National Laboratories.
Udovic says that future work will involve chemically tweaking the hydride’s properties in order to optimize its performance. At this point, it would be necessary to operate a battery above the phase transition temperature, so one goal will be to bring the transition temperature down to as close to room temperature as possible—a goal he is confident is within reach.
“You could probably use this material in a battery right now,” he says. “But the lower the temperature required to make it work, the more useful it will be.”
Reference: “Exceptional Superionic Conductivity in Disordered Sodium Decahydro-closo-decaborate” by Terrence J. Udovic, Motoaki Matsuo, Wan Si Tang, Hui Wu, Vitalie Stavila, Alexei V. Soloninin, Roman V. Skoryunov, Olga A. Babanova, Alexander V. Skripov, John J. Rush, Atsushi Unemoto, Hitoshi Takamura and Shin-ichi Orimo, 13 October 2014, Advanced Materials.
DOI: 10.1002/adma.201403157








This is a good result but not a surprise theoretically. Another element is a good candidate to replace Na.