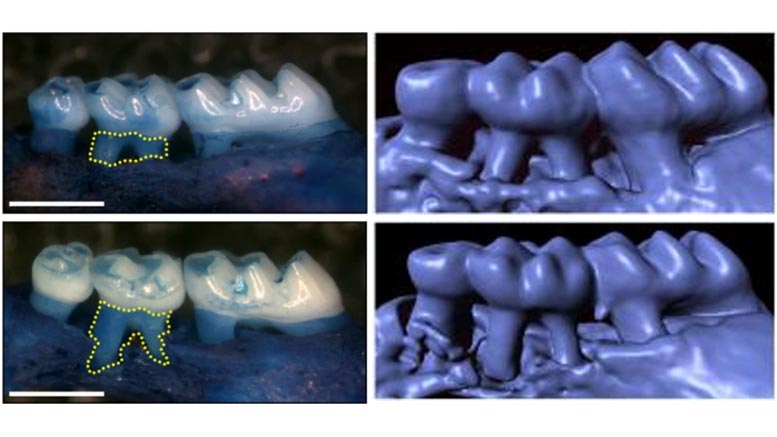
Mice lacking α-gustducin develop accelerated loss of bone supporting the teeth and more severe ligature-induced periodontitis as shown in the left panels and the 3D-rendered computed tomography images (right panels) of maxillae from wild-type (upper) and mice without gustducin (lower). Credit: Monell Chemical Senses Center; Nature Communications
Sentinels in the mouth: Special sensory cells in the gums protect against periodontitis
Newly discovered chemical-sensing cells in the gums protect the mouth by standing guard against infections that damage soft tissue and destroy the bone that supports the teeth, report researchers from the Monell Chemical Senses Center in an animal study published this week in Nature Communications. With the help of bitter taste receptors that also detect byproducts from harmful bacteria, these special gum cells trigger the immune system to control the amount and type of bacteria in the mouth and could one day lead to personalized dental treatments against gum disease.
Periodontitis is a serious gum disorder induced by an imbalance in the bacteria and other microorganisms of the mouth (the oral microbiome). It is the sixth-most prevalent infectious disease and the most common cause of tooth loss worldwide.
Monell Center Director and President Robert Margolskee, MD, PhD and cell biologist Marco Tizzano, PhD, along with colleagues from Sichuan University, found that the newly identified cells, known as solitary chemosensory cells (SCCs), are present in the gums of mice. Here they express several types of taste receptors along with a downstream coupling protein called gustducin. SCCs are taste-like chemical detectors that sense irritants and bacteria, and biologists have found them throughout the gut, urinary tract, nasal cavities, and now in the gums.
“These sensory cells may provide a new approach for personalized treatment of periodontitis by harnessing a person’s own innate immune system to regulate their oral microbiome,” said Margolskee.
The team showed that knocking out taste-signaling molecules like gustducin or genetically removing gum SCCs in the mice leads to the overgrowth of pathogenic oral bacteria and periodontitis. Conversely, stimulating bitter taste receptors in SCCs promotes the production of anti-microbial molecules.
Mice without gustducin in their SSCs have a more damaging set of microbes living in their mouths compared to normal mice, implying that the lack of gustducin disconnects the sentinel cells’ molecular signal to other systems. Importantly, differences in the oral bacterial composition of the gustducin-less mice compared to normal mice occurred before any loss of bone in the gums, implying that differences in the oral microbiome could be used as a harbinger of disease.
In previous studies with other tissues, researchers found that activating the bitter taste receptor TAS2R38, which is expressed in human nasal SCCs, stimulates the secretion of antimicrobial peptides (AMPs) that repress the growth of respiratory pathogens. To examine the effects of repeated stimulation of gum SCCs on periodontitis (and the release of gum AMPs) in the current study, the researchers applied a mouthwash containing the ultra-bitter substance denatonium to the gums of the mice twice a day. This activated gum SCCs as well as their antimicrobial molecules, which reduced periodontitis in normal mice, but not in the periodontitis-susceptible animals without gustducin.
After receiving the denatonium, normal mice showed an enhanced release of an AMP called β-defensin, which was produced at more than twice the levels seen in control mice treated only with saline mouthwash. On the other hand, when the gustducin-less mice were given denatonium mouthwash, there was no effect on the level of β-defensin.
“Our study adds to a growing list of tissues we now know contain SCCs and indicates that the common molecular pathways in gum SCCs are involved in the regulation of oral microbiota,” said Tizzano. “In the absence of taste signaling in the gums, the oral microbiome changed in mice without gustducin.”
From this animal study, and unpublished work in humans, the team expects that gum SCCs in humans play a similar role in regulating the makeup of the oral microbiome, notes coauthor Xin Zheng, a dental science researcher from the National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology. They surmise that since genetic differences in taste receptors are commonly detected in people, particularly loss-of-function of the bitter taste receptor TAS2R38, the dysfunction of taste-receptor-mediated innate immune responses could be used for dental chair-side screening for individuals who may be most susceptible to oral infectious diseases.
###
This work was supported by the National Institute on Deafness and Other Communication Disorders (R01 DC014105, R03 DC012413, R01 DC016598, R01 DC013807), the National Natural Science Foundation of China, and in part, by funding from an NIDCD Core Grant (P30 DC011735) and the National Science Foundation (DBI-0216310). Additional support came from institutional funds from the Monell Center.
Reference: “Gingival solitary chemosensory cells are immune sentinels for periodontitis” by Xin Zheng, Marco Tizzano, Kevin Redding, Jinzhi He, Xian Peng, Peihua Jiang, Xin Xu, Xuedong Zhou & Robert F. Margolskee, 3 October 2019, Nature Communications.
DOI: 10.1038/s41467-019-12505-x




Be the first to comment on "Newly Discovered Chemical-Sensing Cells in the Gums Protect Against Periodontitis"