Researchers at the University of Washington School of Medicine have successfully engineered stem cells that do not cause dangerous arrhythmias, a major complication previously hindering stem-cell therapies for injured hearts. By using CRISPR-based genome editing to modify ion channels in the stem cells, the team created a new line of cells called “MEDUSA,” which engraft in the heart, mature, and integrate into heart muscle without generating dangerous heart rates.
Engineered stem cells do not provoke dangerous heart rhythms, a challenge that has prevented the progress of stem cell transplants for damaged hearts.
Researchers at the University of Washington School of Medicine in Seattle have successfully created stem cells that do not cause dangerous arrhythmias, a complication that has to date thwarted efforts to develop stem-cell therapies for injured hearts.
“We have found what we have to tackle to make these cells safe,” said Silvia Marchiano, a postdoctoral fellow in the laboratory of Chuck Murry at the UW Medicine Institute for Stem Cell and Regenerative Medicine. Marchiano is the lead author of a paper describing the findings published Thursday, April 6, in the journal Cell Stem Cell. The work was done in collaboration with the Seattle company Sana Biotechnology.
In earlier research, Murry’s team employed heart muscle cells derived from stem cells to mend heart tissue injuries resulting from myocardial infarction. This form of heart attack takes place when the blood supply to the cardiac muscle is obstructed, leading to the death of heart cells. Since cardiac cells do not regenerate, the damaged tissue is substituted by scar tissue. This compromises the heart’s strength and hinders its blood-pumping function. Extensive harm can culminate in heart failure and even death.
To create their therapeutic heart cells, the Seattle researchers used pluripotent stem cells. Unlike adult stem cells, which have specialized to become specific cell types, pluripotent stem cells can become any type of cell in the body.

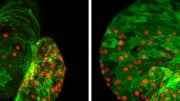
Postdoctoral fellow Silvia Marchiano and research scientist Hans Reinecke look at cardiac stem cells in Chuck Murry’s lab at the UW Medicine Institute for Stem Cell and Regenerative Medicine Research in Seattle. Credit: Michael McCarthy
From 2012 to 2018 the Seattle team successfully injected pluripotent stem cells into damaged heart walls to create new muscle to replace that lost during an infarction. In animal studies, they showed that the grafted cells would integrate with the heart muscle, beat in synchrony with the other heart cells and improve the heart’s contractility. These findings demonstrated that stem cell therapy could potentially be used to rescue damaged hearts.
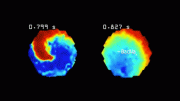
But there was one major complication. During the early weeks of engraftment, the hearts tended to beat at a dangerously high rate. Unless a way could be found to prevent or suppress this problem, stem cells could not become a safe treatment for myocardial infarction and heart failure.
“Our goal is to create working contractile cells that would not try to set their own pace,” Murry said.
In the mature heart, the heart rate is regulated by specialized cells called pacemaker cells. These cells generate electric signals at regular intervals that induce the other heart cells to contract.
In pacemaker cells, the voltage cycles back and forth from negative (hyperpolarized) to positive (depolarized). Murry compares it to a metronome with positive ions swooshing in and out of the cell through these channels. The rate at which this cycle of repolarization and depolarization occurs determines the heart rate.
In early embryonic hearts, however, this system, in which relatively few cells have become specialized pacemaker cells while the rest have become quiescent contractile cells, has not developed. All the cells are pacemakers. Murry and his colleagues suspected that the engrafted stem cells were behaving like early embryonic cells chaotically generating signals and causing the dangerous heart rhythms.
To sort out what was causing these cells to behave this way, the researchers used a technique called RNA sequencing to find out which ion channels were being made at different times as the cells matured. The sequencing revealed that some types of ion channels appear early in development and then disappear as the cell matures while other types of ion channels appear later in development. Like an unfolding mystery, this gave the researchers their list of suspects.
To determine which ion channels were the culprits carrying the arrhythmia-causing current, the scientists used CRISPR-based genome editing to systematically knock out depolarizing genes or to activate repolarizing genes. This proved surprisingly complex. They had hypothesized that there would be a single ion channel causing the arrhythmia, but none of the single-gene edits eliminated the rapid heart rhythms. The researchers then undertook a painstaking process of “playing the combinations” by performing double and triple gene edits. Vexingly, none of these edits eliminated the arrhythmia, and some seemed to make it worse.
Finally, the scientists created a stem cell line in which three depolarizing genes were knocked out and one repolarizing gene was activated. That did the trick. Cardiac muscle cells generated from these stem cells were electrically quiescent, like adult heart muscle, but they contracted when given an electrical signal to mimic a natural pacemaker. The researchers termed these cells “MEDUSA” (for modifying electrophysiological DNA to understand and suppress arrhythmias). The MEDUSA cardiomyocytes engraft in the heart, mature into adult cells, electrically integrate into heart muscle, and beat in sync with natural pacemaking, all without generating dangerous heart rates. This, Murry says, is the sine qua non for heart regeneration.
Murry cautions that additional testing with the engineered cells will need to be done, but, he adds, “I think we’ve overcome the biggest roadblock to regenerating the human heart.”
Reference: “Gene editing to prevent ventricular arrhythmias associated with cardiomyocyte cell therapy” by Silvia Marchiano, Kenta Nakamura, Hans Reinecke, Lauren Neidig, Michael Lai, Shin Kadota, Filippo Perbellini, Xiulan Yang, Jordan M. Klaiman, Leslie P. Blakely, Elaheh Karbassi, Paul A. Fields, Aidan M. Fenix, Kevin M. Beussman, Anu Jayabalu, Faith A. Kalucki, Akiko Futakuchi-Tsuchida, Gerhard J. Weber, Sarah Dupras, Hiroshi Tsuchida, Lil Pabon, Lili Wang, Björn C. Knollmann, Steven Kattman, R. Scott Thies, Nathan Sniadecki, W. Robb MacLellan, Alessandro Bertero and Charles E. Murry, 6 April 2023, Cell Stem Cell.
DOI: 10.1016/j.stem.2023.03.010
The study was funded by the UW Medicine Heart Regeneration Program, the Washington Research Foundation, Mike and Lynn Garvey, Sana Biotechnology, the National Institutes of Health, the Fondation Leducq Transatlantic Network of Excellence, and a Bruce-Laughlin Research Fellowship.



Collection of Cells from Uterus without Surgery or causing any damage at all, if possible from Uterus Every Month for 9 Months may help Baby and mother even in distant future. (Of course, Value of this procedure must first be discerned by working on Various Animal sp.) Who knows? It may be a Treasure. Also, Instead of letting Human Corpses to disintegrate underneath the concrete tombs, they have to be preserved at Absolute Temperature beneath the Alaskan Snow Mountains. Amazon collects and sells various items to the world nowadays. It was a waste before. But, different in the Modern world after Deciphering DNA Structure in 1953, protein synthesis from DNA to mRNA to tRNA etc., AND Recent CRISPR Gene Editing ability, which can only be used safely in future.
After DNA, Salute to Minute Ribosome Particles in Cytoplasm that bind mRNA and tRNA to Synthesize Proteins using 20 Aminoacids.