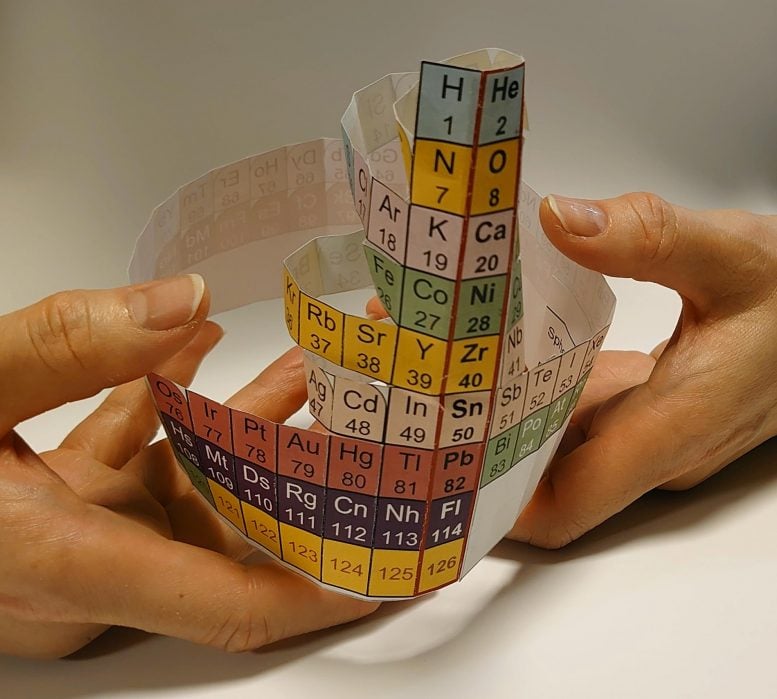
Protons have different stable magic numbers: 2, 8, 20, 28, and so on. When nuclear orbits are filled with protons, they form stable nuclei, analogous to the noble-gas elements. Credit: Kyoto University/Yoshiteru Maeno/Kouichi Hagino
A staple in every science classroom is the periodic table of elements, and for many it is their first introduction to the vast mysteries of the natural world.
Now physicists from Kyoto University have unveiled a new table that provides a different perspective on the building blocks of the universe. While the traditional table is based on the behavior of electrons in an atom, this new table is based on the protons in the nucleus.
“The periodic table of the elements is one of the most significant achievements in science, and in its familiar form it is based on the shell structure of electron orbitals in atoms,” explains Yoshiteru Maeno, one of the co-developers of the new table.
“But atoms are comprised of two types of charged particles that designate each element: electrons orbiting the core and protons in the core itself.”
The team’s new ‘Nucletouch’ table — also available as a 3D model — was announced recently in the journal Foundations of Chemistry.
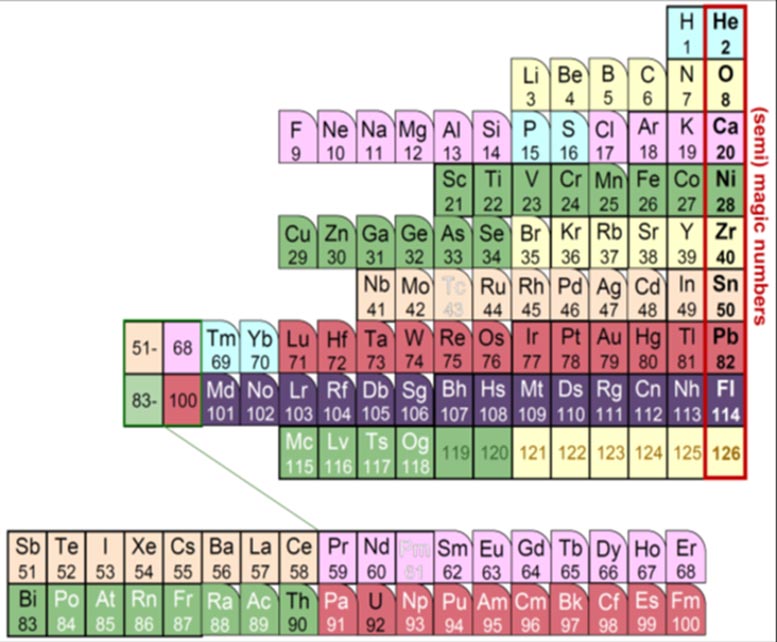
The fundamental elements organized by their proton ‘magic number’. Credit: Kyoto University/Yoshiteru Maeno/Kouichi Hagino
Over 150 years have passed since Dmitri Mendeleev discovered the periodic law that lead him to propose the classic periodic table. He even had the foresight to add space for elements that were still unknown in his time.
“Fundamentally, it comes down to the electrons in each atom. Atoms are considered to be stable when electrons completely fill their ‘shell’ of orbits around the nucleus,” continues Maeno.
“So-called ‘noble gases’, inert elements such as helium, neon, and argon, rarely react with other elements. Their most stable electron numbers are 2, 10, 18, 36, and so on.”
Maeno describes these as atomic ‘magic numbers’, and importantly the same principle can also be applied to protons. Imagining that protons in a nucleus exist in ‘orbits’ may seem like a stretch, but the discovery of the concept was awarded the 1963 Nobel prize in physics.
Protons have different stable magic numbers: 2, 8, 20, 28, and so on. Among these are familiar elements such as helium, oxygen, and calcium. The Nucletouch table places these ‘magic nuclei’ at its center, providing a new perspective on the elements.
“Similar to electrons, when nuclear orbits are filled with protons, they form stable nuclei, analogous to the noble-gas elements,” says collaborator Kouichi Hagino.
“In our nuclear periodic table, we also see that nuclei tend to be spherically-shaped near the magic numbers, but deformed as you move away from them.”
The team made the table to highlight alternative ways to illustrate the laws of nature, and hopes that enthusiasts and academics alike will find something to enjoy and learn from this fresh new look at an old friend.
Reference: “A nuclear periodic table” by K. Hagino and Y. Maeno, 21 April 2020, Foundations of Chemistry.
DOI: 10.1007/s10698-020-09365-5








Well about time this happenned. Proton based is great
How about extending the thinking to Neutron based Periodic Table — Not a Charged Particle and then further into the Quantum Zone and the various Particles we have discovered both Charged and without any Charges and the field of Quantum Chemistry …..where thetime span of stable existenceis limited and the time dimension of the Space Time Continuum is different as far as stability is concerned.
Maybe some new insights may become visible?
Where are you Sekar Vedaraman? Looking for you for Campion School Classof 1971
ASHIQ PATEL
Yet only taking into account a table that organizes by only one aspect of the entire will still not give a full picture.
A more broad based understanding of behavior of elemental forces and parts concerning matter and the space that lies between the particles.
Teaching the principles of individual aspects, protons, neurons, electrons, ionized States; with relation to stability. Into the quantum realm of spooky interaction and how energy and perception effect the natural world around us
Another load on the already overloaded curriculum of the schools. Even if they include it in curriculum and it becomes used widely kindly delete everything from the syllabus and don’t teach the useless history
Under new normal new knowledge will be discovered
Apart from isotopic stability, what other property does this ordering clarify ? When Mendelyev made his table, he had no idea of electrons, protons or neutrons. The ordering was based on atomic weights taking chemical properties into account. The long form of the Periodiс Table also clarifies Chemical properties.