A collaboration between scientists at Cambridge and University College London has led to the discovery of a new form of ice that more closely resembles liquid water than any other and may hold the key to understanding this most famous of liquids.
The new form of ice is amorphous. Unlike ordinary crystalline ice where the molecules arrange themselves in a regular pattern, in amorphous ice, the molecules are in a disorganized form that resembles a liquid.
“Our discovery of MDA raises many questions on the very nature of liquid water and so understanding MDA’s precise atomic structure is very important.” — Dr. Michael Davies
In their paper, published in the journal Science on February 2, the team created a new form of amorphous ice in an experiment and achieved an atomic-scale model of it in a computer simulation. The experiments used a technique called ball-milling, which grinds crystalline ice into small particles using metal balls in a steel jar. Ball-milling is regularly used to make amorphous materials, but it had never been applied to ice.
The team found that ball-milling created an amorphous form of ice, which unlike all other known ices, had a density similar to that of liquid water and whose state resembled water in solid form. They named the new ice medium-density amorphous ice (MDA).
To understand the process at the molecular scale the team employed computational simulation. By mimicking the ball-milling procedure via repeated random shearing of crystalline ice, the team successfully created a computational model of MDA.
“Our discovery of MDA raises many questions on the very nature of liquid water and so understanding MDA’s precise atomic structure is very important,” said co-author Dr. Michael Davies, who carried out the computational modeling. “We found remarkable similarities between MDA and liquid water.”
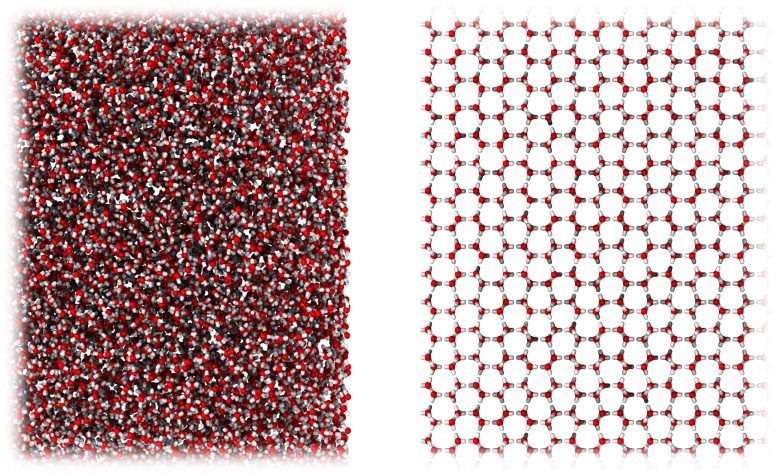
A new form of ice very similar in molecular structure to liquid water (left), compared to ordinary crystalline ice (right). Credit: University of Cambridge
A happy medium
Amorphous ices have been suggested to be models for liquid water. Until now, there have been two main types of amorphous ice: high-density and low-density amorphous ice.
As the names suggest, there is a large density gap between them. This density gap, combined with the fact that the density of liquid water lies in the middle, has been a cornerstone of our understanding of liquid water. It has led in part to the suggestion that water consists of two liquids: one high- and one low-density liquid.
Senior author Professor Christoph Salzmann said: “The accepted wisdom has been that no ice exists within that density gap. Our study shows that the density of MDA is precisely within this density gap and this finding may have far-reaching consequences for our understanding of liquid water and its many anomalies.”
A high-energy geophysical material
The discovery of MDA gives rise to the question: where might it exist in nature? Shear forces were discovered to be key to creating MDA in this study. The team suggests ordinary ice could undergo similar shear forces in the ice moons due to the tidal forces exerted by gas giants such as Jupiter.
Moreover, MDA displays one remarkable property that is not found in other forms of ice. Using calorimetry, they found that when MDA recrystallizes to ordinary ice it releases an extraordinary amount of heat. The heat released from the recrystallization of MDA could play a role in activating tectonic motions. More broadly, this discovery shows water can be a high-energy geophysical material.
Professor Angelos Michaelides, lead author from Cambridge’s Yusuf Hamied Department of Chemistry, said: “Amorphous ice in general is said to be the most abundant form of water in the universe. The race is now on to understand how much of it is MDA and how geophysically active MDA is.”
For more on this research, see New Form of Ice Discovered.
Reference: “Medium-density amorphous ice” by Alexander Rosu-Finsen, Michael B. Davies, Alfred Amon, Han Wu, Andrea Sella, Angelos Michaelides and Christoph G. Salzmann, 2 February 2023, Science.
DOI: 10.1126/science.abq2105











It seems possible shear forces are drawing out the hydrogen bonds and randomly re-anchoring their Van der Waals couplings to the oxygen of more distant molecules.
Mentioned before that I had an idea about protons becoming retro-reflective to gravity flows a few years ago; it implied protons (and neutrons) can concentrate the energy carried with such information flows. As hydrogen ions are single protons, spinning bound hydrogen ions would show this cold-focusing effect best as the spins of other bound ions impart positional oscillations in their nucleons, interfering with the focus except between atoms spinning at identical rates on a common axis. This cold proton effect would strengthen and lengthen the Van der Waals bonds for any atoms bound to hydrogen, compared to conventional predictions.
One way to visualize this cold gravity focus effect, which is to imagine the effect of cooling on a nucleon’s gravitational reflectivity, is with a model formed by a reflective balloon enveloping three mutually intersecting disks. As the temperature drops the air volume in the balloon drops with it until it resembles a triaxial omni-retroreflector used by small boats for radar visibility.
With ball milling it’s possible to exploit fracturing and plasticity or ductility. Ductility/plasticity is the more interesting and non-intuitive modality to me here. With partial ductility there is also some elasticity possible, and the repetitiveness of milling may thus allow for some bond annealing to occur, I imagine.
From the description this process seems to be creating finely grained particles of normal ice. I’m sure I’m misunderstanding something, but “(the process) grinds crystalline ice into small particles using metal balls in a steel jar…” sounds like the production of sand or some other granular, non-liquid matter that behaves in accord with fluid dynamics.
So they discovered slush?
Don’t tell them that convenience stores and amusement parks have been selling slushies for decades.
They’re slushies close to liquid water density, at STP; and they let some recrystallize as ice, which got warmer then. So…off to find if any amorphous ice, glacial, space or otherwise, is this phase and to make use of the phase change if possible.