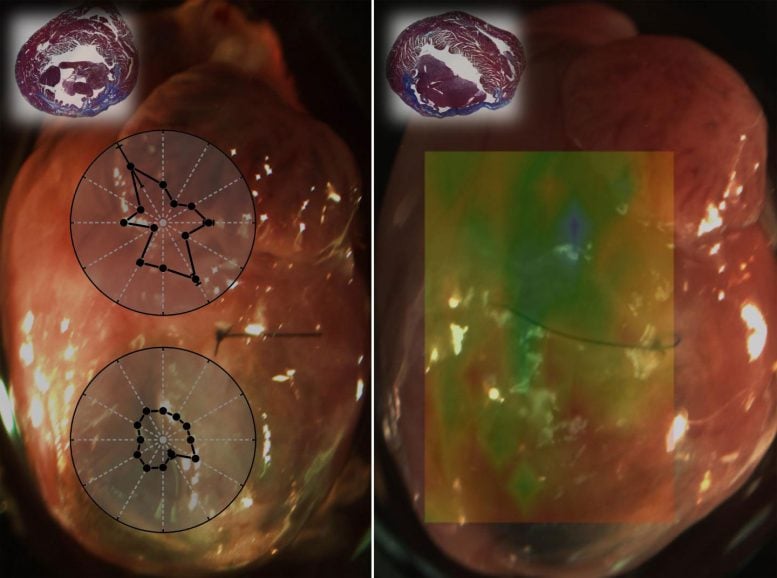
Researchers used optical coherence elastography for biomechanical assessment of tissue damage caused by a heart attack in mice. The overlays in the left image show direction-dependent differences between healthy (top) and damaged tissue (bottom). The overlay in the right image show spatially resolved tissue stiffness measurements obtained with OCE. The green shows healthy region while the yellow, red areas are damaged regions with decreased elasticity. Credit: Kirill V. Larin of University of Houston
Researchers have developed a new way to capture the detailed biomechanical properties of heart tissue. The high-resolution optical technique fills an important technology gap necessary to develop and test therapies that might eventually be used to heal heart damage after a heart attack.
“Today about one million people suffer heart attacks every year, and there is currently no cure for the resulting cardiac tissue scarring,” said Kirill V. Larin of University of Houston, Texas who co-led the research with James F. Martin from the Baylor College of Medicine and the Texas Heart Institute. “We are working to develop ways to regenerate heart tissue and our research works to measure the mechanical properties to determine if the heart is healing in response to therapies.”
In The Optical Society (OSA) journal Biomedical Optics Express, the researchers report results from studies in mice showing that a high-resolution technique known as optical coherence elastography (OCE) can be used to compare the mechanical properties of healthy tissue and tissue scarred by an induced heart attack. The researchers plan to use the technique to evaluate the effectiveness of therapies aimed at reversing damage to heart tissue.
Regenerating heart tissue
Heart attacks occur when a blood clot prevents the coronary artery from delivering oxygen-rich blood to the heart. This blockage deprives the heart muscle of oxygen and within a short time causes permanent damage in the form of scarred tissue. This damage takes away energy from the beating heart and affects how well it can contract to pump blood.
“Experiments have shown that tissue from newborn mammalian hearts can completely regenerate, but with age this regeneration capability diminishes,” said Larin. “Martin’s group is working on ways to manipulate these molecular pathways in a way that stimulates the adult heart tissue to repair itself.”
The researchers turned to OCE, a technique developed in Larin’s lab, to see if it might be useful for observing how well experimental therapies worked in mouse models. OCE is based on the biomedical imaging technique optical coherence tomography (OCT), which can provide high-resolution images of the microstructures of tissue. However, rather than obtaining structural information, OCE uses the principles of OCT to create high-resolution maps of tissue mechanics.
OCE is ideal for observing the tissue mechanics in mouse hearts because it has the resolution necessary to detect whether the boundary between healthy and scarred tissue moves in response to therapy. Although other imaging modalities such as MRI or ultrasound can be used to examine tissue mechanics, they are better suited for large areas of tissue rather than a small, delicate mouse heart.
Performing OCE requires mechanical waves to be inducted in the tissue. Much like a stone dropped into water causes a pattern of waves, tissue exposed to a small mechanical force will exhibit a specific pattern of waves propagating through it. The researchers developed analytical models to reconstruct the mechanical properties of the tissue by analyzing either the speed or spatial characteristics of the waves.
“Because of the small size and delicate nature of the mouse heart, we had to make special equipment to generate very small perturbations on the tissue,” said Larin. “The pressure, timing, and location of this applied force had to be very precise. The waves also had to have very small amplitudes, which was important for preserving the tissue.”
Examining tissue after a heart attack
The researchers tested their imaging approach in tissue samples from mice. After an induced heart attack, the mice developed scarring that would be similar to that caused by a heart attack in people. At six weeks, the researchers excised the hearts and used OCE to measure the mechanical properties of the heart tissue.
The researchers saw that the damaged tissue showed decreased anisotropy – or directionally of the wave propagation – compared to healthy tissue. This observation indicated that the muscle fibers in the damaged area were more disorganized than healthy tissue. They also saw differences in tissue stiffness between healthy and damaged tissue using OCE.
“This is the first application of OCE for high-resolution mapping of muscle mechanical properties of the heart,” said Larin. “We were able to see differences in the mechanical properties of normal heart tissue and areas with myocardial infarction. In the future, we want to use the technique to examine regenerated heart tissue to help us find a therapy that can benefit the millions of people worldwide who have experienced a heart attack.”
Reference: “Biomechanical assessment of myocardial infarction using optical coherence elastography” by Shang Wang, Manmohan Singh, Thuy Tien Tran, John Leach, Salavat R. Aglyamov, Irina V. Larina, James F. Martin, and Kirill V. Larin, 23 January 2018, Biomedical Optics Express.
DOI: 10.1364/BOE.9.000728




Hi
Are there any proven therapies, that repair post heart attack scar tissue? Or trial in progress