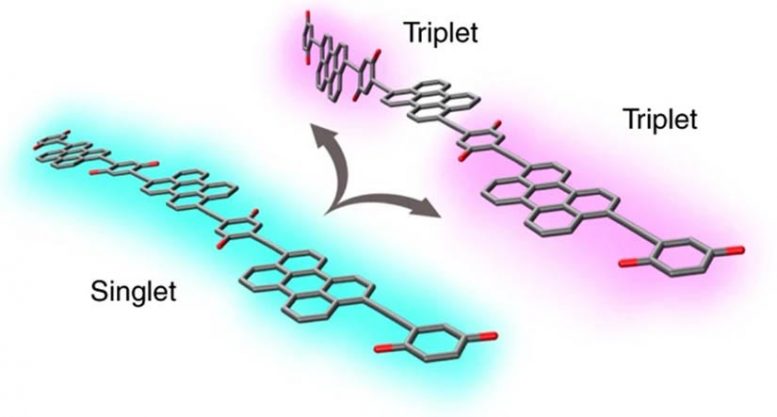
By twisting when excited, some long chains of organic molecules can isolate triplet excitons at opposite ends of the molecule. Credit: NREL
In the twisting and turning of long organic molecules, National Renewable Energy Laboratory (NREL) researchers have found a promising group of materials for tomorrow’s super-efficient solar cells.
In a new paper in Nature Chemistry, NREL researchers demonstrated how a carefully designed molecule can efficiently split the energy imparted by one photon into two excited states and keep them separated for several microseconds—a long time at the molecular scale. The three authors—Nadia Korovina, Chris Chang, and Justin Johnson—drew on their diverse expertise in chemistry and computer modeling to design this new molecule and learn how it functions.

Postdoctoral researcher Nadia Korovina synthesized the new molecules. After completing her postdoctoral work at NREL, she has become a professor at California State University, Chico. Credit: Kurt Van Allsburg, NREL
When a photon strikes an appropriate semiconductor material, it creates an exciton—an excited energy state. In some organic molecules, the exciton can split, forming two triplet excitons. This process of “singlet fission” could potentially be used to extract more energy from each absorbed photon than in a traditional solar cell. However, if these two triplets encounter each other, they will recombine and cease to exist. Additionally, the process by which a singlet splits into two stable triplets can often lose some energy to heat.
An ideal organic photovoltaic molecule would address both these issues—meaning it efficiently converts singlet excitons into triplets with no heat loss and keeps those triplets separate so they cannot recombine. Rather than search for such a molecule, the NREL team decided to design their own. Drawing on previous research, the team knew in general what types of organic molecules showed promise. But they needed to determine exactly how long and complex these molecules should be to prevent triplet recombination.
With that objective in mind, Korovina synthesized a series of molecules of varying length, all built of chains of chromophores—light-absorbing molecular building blocks.
“The most difficult part was designing molecules in which the fine balance of singlet and triplet energies was achieved,” Korovina said. “After about a year of trial and error, we had the right molecules from which we were able to learn the intricacies of the singlet fission process.”
After carefully sorting these molecules by size, the team found that a chain of at least three chromophores is needed to successfully isolate two triplet excitons.
To figure out exactly how the chain of chromophores was isolating the two triplets, Johnson and Korovina turned to Chang, a computational scientist with a background in biochemistry. “I see modeling as helping to answer two big questions,” Chang said. “How does it work based on underlying principles? And what does it look like when it does so?”
By creating and then refining a model of how the molecules move and interact, the team discovered that a twisting motion gives the molecules the characteristics needed to isolate the triplets. The molecular chain is usually floppy and flexible when not under illumination; but when it absorbs a photon, the chain twists around its central axis and initially stiffens, resulting in a shape that facilitates the formation of two triplets. The subsequent twisting that occurs after the initial process finishes helps to spatially separate the two triplets, lengthening their lifespans.
By combining experimental and modeling approaches, the team was not only able to develop a promising energy-absorbing molecule, but also to explain its function in detail. Now that the fundamental mechanism is well understood, future development and use of similar molecules in high-efficiency solar cells or other photoelectrochemical systems should be easier.
“New discoveries like this are possible without crossing disciplines,” Johnson said, “but combining expertise like we did can yield a much bigger impact.”
Reference: “Spatial separation of triplet excitons drives endothermic singlet fission” by Nadezhda V. Korovina, Christopher H. Chang and Justin C. Johnson, 2 March 2020, Nature Chemistry.
DOI: 10.1038/s41557-020-0422-7







Be the first to comment on "Two-for-One Energy From Photons: Tomorrow’s Super-Efficient Solar Cells"