
Artist’s depiction of a Mars exploration base
A new study outlines a biotechnology process to produce rocket fuel on red planet.
Researchers at the Georgia Institute of Technology have developed a concept that would make Martian rocket fuel, on Mars, that could be used to launch future astronauts back to Earth.
The bioproduction process would use three resources native to the red planet: carbon dioxide, sunlight, and frozen water. It would also include transporting two microbes to Mars. The first would be cyanobacteria (algae), which would take CO2 from the Martian atmosphere and use sunlight to create sugars. An engineered E. coli, which would be shipped from Earth, would convert those sugars into a Mars-specific propellant for rockets and other propulsion devices. The Martian propellant, which is called 2,3-butanediol, is currently in existence, can be created by E. coli, and, on Earth, is used to make polymers for production of rubber.
The process is outlined in a paper, “Designing the bioproduction of Martian rocket propellant via a biotechnology-enabled in situ resource utilization strategy,” published in the journal Nature Communications.

A photo of Mars’ Jezero Crater, taken by NASA’s Perseverance Mars rover. Credit: NASA/JPL-Caltech/ASU/MSSS
Rocket engines departing Mars are currently planned to be fueled by methane and liquid oxygen (LOX). Neither exist on the red planet, which means they would need to be transported from Earth to power a return spacecraft into Martian orbit. That transportation is expensive: ferrying the needed 30 tons of methane and LOX is estimated to cost around $8 billion. To reduce this cost, NASA has proposed using chemical catalysis to convert Martian carbon dioxide into LOX, though this still requires methane to be transported from Earth.
As an alternative, Georgia Tech researchers propose a biotechnology based in situ resource utilization (bio-ISRU) strategy that can produce both the propellant and LOX from CO2. The researchers say making the propellant on Mars using Martian resources could help reduce mission cost. Additionally, the bio-ISRU process generates 44 tons of excess clean oxygen that could be set aside to use for other purposes, such as supporting human colonization.

“You need a lot less energy for lift-off on Mars, which gave us the flexibility to consider different chemicals that aren’t designed for rocket launch on Earth.” — Pamela Peralta-Yahya. Credit: Georgia Tech
“Carbon dioxide is one of the only resources available on Mars. Knowing that biology is especially good at converting CO2 into useful products makes it a good fit for creating rocket fuel,” said Nick Kruyer, first author of the study and a recent Ph.D. recipient from Georgia Tech’s School of Chemical and Biomolecular Engineering (ChBE).
The paper outlines the process, which begins by ferrying plastic materials to Mars that would be assembled into photobioreactors occupying the size of four football fields. Cyanobacteria would grow in the reactors via photosynthesis (which requires carbon dioxide). Enzymes in a separate reactor would break down the cyanobacteria into sugars, which could be fed to the E. coli to produce the rocket propellant. The propellant would be separated from the E. coli fermentation broth using advanced separation methods.
The team’s research finds that the bio-ISRU strategy uses 32% less power (but weighs three times more) than the proposed chemically enabled strategy of shipping methane from Earth and producing oxygen via chemical catalysis.
Because the gravity on Mars is only one-third of what is felt on Earth, the researchers were able to be creative as they thought of potential fuels.
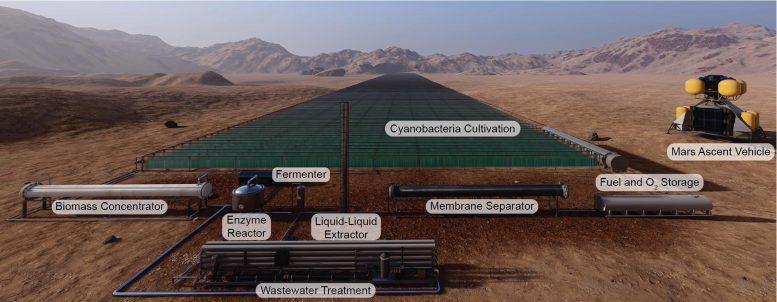
Photobioreactors the size of four football fields, covered with cyanobacteria, could produce rocket fuel on Mars. Credit: BOKO mobile study
“You need a lot less energy for lift-off on Mars, which gave us the flexibility to consider different chemicals that aren’t designed for rocket launch on Earth,” said Pamela Peralta-Yahya, a corresponding author of the study and an associate professor in the School of Chemistry & Biochemistry and ChBE who engineers microbes for the production of chemicals. “We started to consider ways to take advantage of the planet’s lower gravity and lack of oxygen to create solutions that aren’t relevant for Earth launches.”
“2,3-butanediol has been around for a long time, but we never thought about using it as a propellant. After analysis and preliminary experimental study, we realized that it is actually a good candidate,” said Wenting Sun, associate professor in the Daniel Guggenheim School of Aerospace Engineering, who works on fuels.
The Georgia Tech team spans the campus. Chemists, chemical, mechanical, and aerospace engineers came together to develop the idea and process to create a viable Martian fuel. In addition to Kruyer, Peralta-Yahya, and Sun, the group included Caroline Genzale, a combustion expert and associate professor in the George W. Woodruff School of Mechanical Engineering, and Matthew Realff, professor and David Wang Sr. Fellow in ChBE, who is an expert in process synthesis and design.
The team is now looking to perform the biological and materials optimization identified to reduce the weight of the bio-ISRU process and make it lighter than the proposed chemical process. For example, improving the speed at which cyanobacteria grows on Mars will reduce the size of the photobioreactor, significantly lowering the payload required to transport the equipment from Earth.
“We also need to perform experiments to demonstrate that cyanobacteria can be grown in Martian conditions,” said Realff, who works on algae-based process analysis. “We need to consider the difference in the solar spectrum on Mars both due to the distance from the Sun and lack of atmospheric filtering of the sunlight. High ultraviolet levels could damage the cyanobacteria.”
The Georgia Tech team emphasizes that acknowledging the differences between the two planets is pivotal to developing efficient technologies for the ISRU production of fuel, food, and chemicals on Mars. It’s why they’re addressing the biological and material challenges in the study in an effort to contribute to the goal of future human presence beyond Earth.
“The Peralta-Yahya lab excels at finding new and exciting applications for synthetic biology and biotechnology, tackling exciting problems in sustainability,” added Kruyer. “Application of biotechnology on Mars is a perfect way to make use of limited available resources with minimal starting materials.”
Reference: “Designing the bioproduction of Martian rocket propellant via a biotechnology-enabled in situ resource utilization strategy” by Nicholas S. Kruyer, Matthew J. Realff, Wenting Sun, Caroline L. Genzale and Pamela Peralta-Yahya, 25 October 2021, Nature Communications.
DOI: 10.1038/s41467-021-26393-7
The research was supported by a NASA Innovative Advanced Concepts (NIAC) Award.










So–what planet or large moon is that supposed to be in the Martian sky?
Ha! It was there in the stock image, and I was going to edit it out for realism. Then I thought, “it looks cool and no one will notice.” (Or maybe I was lazy.)
I should have edited the image.
Adding a feedhorn would make it look like an oversized dish on a gimbal.
Is that an Apache with wheels?
So, after all the years of effort that NASA has put into trying NOT to infect Mars with Earth life forms we’re now proposing to deliberately infect it? That’s a bit of a policy change…
Wouldn’t it make even more sense to develop and store such fuels on the moon given it has even less gravitational pull than Mars? That way, rockets going from Earth to Moon won’t have to be as heavy, or said another way, we can save the cost of transporting fuel to the Moon for the return trips to Earth, thus making the creation of a colony on the Moon much cheaper.
No carbon dioxide or water on the moon to use in making the fuel.
https://www.universityofcalifornia.edu/news/making-methane-mars
Among the many challenges with a Mars voyage, one of the most pressing is: How can you get enough fuel for the spacecraft to fly back to Earth? Houlin Xin, an assistant professor in physics and astronomy, may have found a solution. He and his team have discovered a more efficient way of creating methane-based rocket fuel on the surface of Mars, which can make the return trip all more feasible.
“The goal is to use in-situ resource utilization to create more fuel. The teams would take water and carbon dioxide to make liquid oxygen and methane. The carbon dioxide would come from the atmosphere, and the water would come from ice reserves. The team uses electrolysis to split water into oxygen and hydrogen, and uses the Sabatier process to take carbon dioxide and hydrogen and create water and methane.”
– From 2019 Inverse article (https://www.inverse.com/article/60133-spacex-how-elon-musk-plans-to-power-mars-space-age-fuel-depots)
Can’t they simply produce hydrogen and oxygen by electrolysing the water ice that is found in abundance on Mars? I though Robert Zubrin came up with this idea years ago.
The most likely first rocket engine to be launching back from Mars is the SpaceX Raptor which is fueled with LOX and methane. SpaceX plans to make the methane on Mars using water and CO2 using the Sabatier process. And of course LOX will be made by electrolysis of water. This article actually mentions making LOX from CO2 which I hope is a misprint. Use of a different fuel rather than methane would require the development of a totally new rocket engine. I wonder who is going to pay for that?