
Sodium Chloride Crystals Microscopic View
Any cook worth their salt knows that a dash of the stuff — which consists mostly of the compound sodium chloride — will dissolve when dropped into a pot of even room-temperature water.
But as a chemist who has spent decades researching how substances behave when confined to infinitesimal spaces, Nebraska’s Xiao Cheng Zeng also knows that what happens at the macroscale does not necessarily hold at the nanoscale.
Zeng and his colleagues recently ran computer simulations to determine how sodium chloride and its salty cousin, lithium chloride, might respond when submerged in a nanoscopic stream of water bordered by two smooth, water-repellent walls.
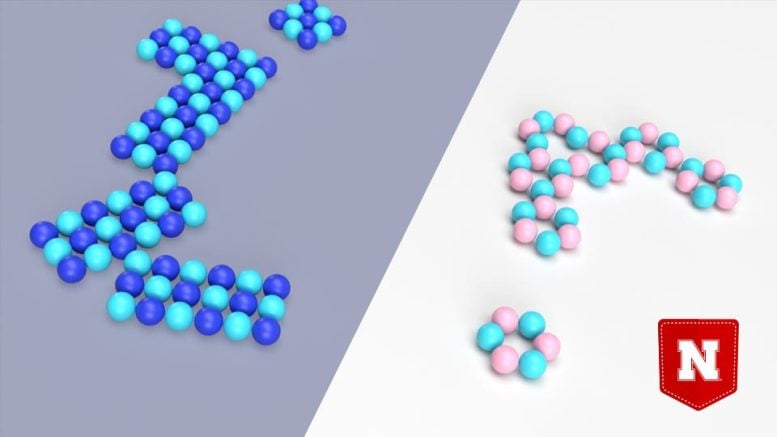
An atomic-level rendering of sodium chloride (left), the primary ingredient in table salt, and lithium chloride (right). New research from Nebraska’s Xiao Cheng Zeng and others has suggested that, when confined to a nanoscopic space, sodium (dark blue) and chlorine (light blue) atoms can reassemble after being dissolved. Lithium (pink) and chlorine atoms can do the same, according to the team’s simulations. Credit: University of Nebraska–Lincoln
Those simulations predicted something wildly counterintuitive. After initially dissolving in the water, the charged, randomly dispersed atoms of both sodium and lithium chloride would spontaneously reassemble into 2D layers, according to the simulations. In the case of sodium chloride, that layer would be identical to its solid, pre-dissolved state: a crystalline pattern of squares, with each sodium atom surrounded by four chlorine atoms, or vice versa. For lithium chloride, the layer would comprise hexagonal rings — three lithium atoms, three chlorine — or zigzagging chains of the atoms, or both.
Based on the team’s calculations, the unexpected behavior emerges partly because nanoscale confinement reduces the interaction strength between a charged atom — sodium, lithium, or chlorine — and the water molecules that typically form a shell around it. That hydration shell normally keeps oppositely charged particles, such as sodium and chlorine, from reassembling after dissolving — but not when confined to a nanoscopic space, the researchers found.
Zeng and his fellow computational chemists hope their predictions will encourage other researchers to conduct experiments that validate or challenge their simulations.
Those predictions might eventually inform the design of nanofluidic devices that transport charged atoms to recreate neuronal activity, Zeng said.
Reference: “Two-dimensional monolayer salt nanostructures can spontaneously aggregate rather than dissolve in dilute aqueous solutions” by Wenhui Zhao, Yunxiang Sun, Weiduo Zhu, Jian Jiang, Xiaorong Zhao, Dongdong Lin, Wenwu Xu, Xiangmei Duan, Joseph S. Francisco and Xiao Cheng Zeng, 23 September 2021, Nature Communications.
DOI: 10.1038/s41467-021-25938-0






“… the charged, randomly dispersed atoms of both sodium and lithium chloride would spontaneously reassemble into 2D layers, …” and, “… nanoscale confinement reduces the interaction strength between a charged atom — sodium, lithium or chlorine …”
In both instances, that should be “ions,” not “atoms!”
You sound really confident in that assessment. However, I’d chalk it up purely to semantics; an ion is but a charged atom after all (or molecule, but in this case atom certainly holds true). Anyone who has taken high-school Chem should intuitively understand where someone is talking about a simple salt like sodium or lithium chloride, the bond is ionic and atoms charged. The very definition of “ion” is “an atom [or molecule] with a net electric charge”.
Yes, I am confident that ionic crystals are created from ions. You have weakened your own argument by acknowledging that sometimes an ion can be a collection of more than one atom.
Yes, it is “semantics,” the meaning of words:
https://www.merriam-webster.com/dictionary/semantics
The whole point of using words that have nuances in their meaning is to avoid having to write a paragraph to explain slight differences such as the difference between an electrically neutral atom and one or more atoms with an electron imbalance leading to a net charge.
Because this is a forum for laymen, and not necessarily scientists, not all readers will have taken high school chemistry. Why not use this forum as a way to educate those who didn’t take chemistry in school?