
A new hydrogen fuel cell is not only the world’s most durable[1] to date, but is also more cost-effective, paving the way for a wider application of green energy in the pursuit of a carbon-neutral world.
Hydrogen fuel cells are a promising clean energy option as they efficiently generate power by converting hydrogen and oxygen into electricity, with zero emission of carbon dioxide, particulate matter, and other air pollutants that may cause smog and other health problems. Hydrogen fuel cells have yet to be widely commercialized, despite their environmental benefits and years of development. That is because its power generation depends heavily on an electrocatalyst — which is largely comprised of the very expensive and rare metal platinum.
Researchers have strived to develop alternatives by replacing platinum with more common and inexpensive materials like iron-nitrogen-carbon. However, those materials have either proven inefficient in power generation or have suffered from poor durability.
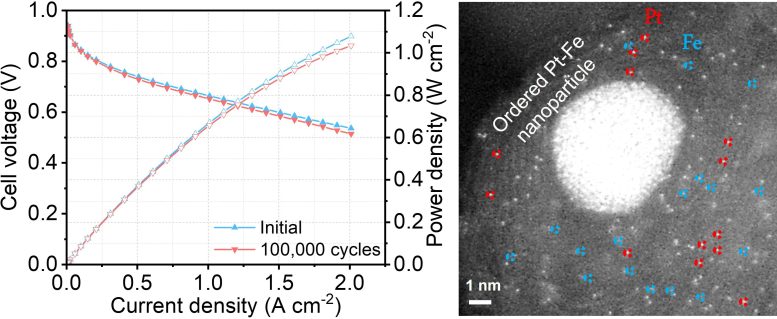
(Left) The new hybrid catalyst maintains the platinum catalytic activity at 97% after 100,000 cycles of accelerated stress test; (Right) The new electrocatalyst contains atomically dispersed platinum, iron single atoms and platinum-iron nanoparticles. Credit: HKUST
Now, a research team led by Prof. Minhua Shao from the Department of Chemical and Biological Engineering at HKUST, discovered a new formula. It not only cuts down the proportion of platinum used by 80 percent, but it also set a record in terms of the cell’s durability level.

Professor Minhua Shao from HKUST’s Department of Chemical and Biological Engineering and the Director of HKUST Energy Institute holds the prototype of the new hydrogen fuel cell. Credit: HKUST
Despite a low portion of platinum, the new hybrid catalyst developed by the research team managed to maintain the platinum catalytic activity at 97% after 100,000 cycles[2] of accelerated stress test, compared to the current catalyst which normally sees a drop of over 50% in performance after just 30,000 cycles. In another test, the new fuel cell did not show any performance decay after operating for 200 hours.[3]
One reason behind such outstanding performance was the fact that the new catalyst has three different active sites for the reaction, instead of just one in current catalysts. Using a formula containing atomically dispersed platinum, iron single atoms, and platinum-iron nanoparticles, the new mix accelerates the reaction rate and achieves a catalytic activity 3.7 times higher than the platinum itself. Theoretically, the higher the catalytic activity, the greater the power it delivers.
Prof. Shao, also the Director of HKUST Energy Institute, said, “Hydrogen fuel cell is an energy conversion device essential for our aspiration of achieving a carbon neutral world, there is a need to expand its use amidst our fight against climate change. We are delighted to see our research findings bringing this goal a step closer. Thanks to the Government’s Green Tech Fund, we will seek to further refine the catalyst and make it compatible with fuel cell vehicles and other electrochemical devices.”
The study was financially supported by the National Key R&D Program of China, Shenzhen Science and Technology Innovation Committee, and the Research Grant Council of the Hong Kong Special Administrative Region. The research findings were recently published in the journal Nature Catalysis.
Notes
- According to the test protocols of US Department of Energy in assessing the durability of fuel cell.
- One cycle is equivalent to 3 seconds with voltage level at 0.6V, followed by another 3 seconds with voltage level at 0.9V.
- The voltage level is set at 0.6V.
Reference: “Atomically dispersed Pt and Fe sites and Pt–Fe nanoparticles for durable proton exchange membrane fuel cells” by Fei Xiao, Qi Wang, Gui-Liang Xu, Xueping Qin, Inhui Hwang, Cheng-Jun Sun, Min Liu, Wei Hua, Hsi-wen Wu, Shangqian Zhu, Jin-Cheng Li, Jian-Gan Wang, Yuanmin Zhu, Duojie Wu, Zidong Wei, Meng Gu, Khalil Amine and Minhua Shao, 2 June 2022, Nature Catalysis.
DOI: 10.1038/s41929-022-00796-1
Funding: National Key Research and Development Program of China, Shenzhen Science and Technology Innovation Committee, the Research Grant Council of the Hong Kong Special Administrative Region, Innovation and Technology Commission of the Hong Kong Special Administrative Region, Foshan-HKUST Project, Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), Shenzhen Natural Science Fund







“… as they efficiently generate power by converting hydrogen and oxygen into electricity, with zero emission of carbon dioxide, particulate matter, and other air pollutants that may cause smog and other health problems.”
However, they do generate water. If the water is condensed and kept onboard a vehicle, it will reduce the performance and mileage as it accumulates, and it will have to be drained periodically, a maintenance job that people may forget to do. If it isn’t purged regularly during the Winter, there is risk of rupturing the holding tank from freezing. If it is released to the environment, it will contribute to icing on roads. The use of fuel cells in cold climates will be problematic.
Whether used in a mobile or fixed station, if it is released as water vapor, it will contribute to increasing the heat index, making urban living even more intolerable than increasing temperature.
Society needs to look at these issues as a system problem, not just an isolated chemical problem.
You can generate water by pissing too, lol. In any case I am hoping the water produced is potable; I live out in the country and my well is running dry, so I need a second source.
How do you suck
You don’t “generate” water by urinating. You move it from one location to another by passing it through your body.
In theory, the water produced by a fuel cell should be pure. However, the air used to provide oxygen may be contaminated with smog and aerosols, and the car itself may introduce heavy metals and lubricants if not designed properly. Try it on your house plants before you try drinking it.
Clyde, then dump the condensate. Where is it written that you need to condense and accumulate the water? Just let it out!
That is an option. However, that means everyone will be driving on wet pavements all the time. It is well known that accidents increase when it is raining. It also means that near sunrise and sunset the blinding glare from the sun will be worse than with dry pavement. The evaporating water will increase the relative humidity, increasing the Heat Index, and exacerbating the Urban Heat Island effect.
In the Winter, that water will freeze on the pavement making black ice, and again increasing the accident rate.
I covered some of these issues in my original comment. Did you not read it all?
Using hydrogen as fuel for land/sea/air vehicles, or for storing energy, or for heating homes, is extremely bad idea,
since it is highly explosive! Seriously thinking there will be never any leak/ruptures/mishandling to trigger massive explosions?
Not to mention there is no need to use hydrogen for anything!
All light vehicles are already becoming electric & all heavy/big land/sea/air (diesel) vehicles
(like trucks & trains & construction/mining/agriculture/military vehicles & ships & aircraft)
just need us to start producing biodiesel at large scales from all possible industrial/agricultural/forestry waste/biomass & even trash & sewage!
For storing energy, there are already grid-size battery solutions!
For heating homes, just produce electricity from solar & wind & nuclear!
(But also upgrade electric grids of all cities/towns, so that they can handle the full load,
even if everybody uses electricity (at the same time) for heating & cooking & charging electric vehicles!)
You are totally misinformed, or you own lithium stocks. When Hydrogen is released and burned it shoots straight upward, as it is far lighter than air. Also, it burns very fast so it is almost over before you know it. Lithium fires, on the other hand, create this smoldering chemical puddle of fire, which reacts violently with water. A lithium fire is the worst nightmare of fire departments. Also don’t forget the fact that a hydrogen vehicle does not need the dirty mining business, and its abuse of peoples worldwide.