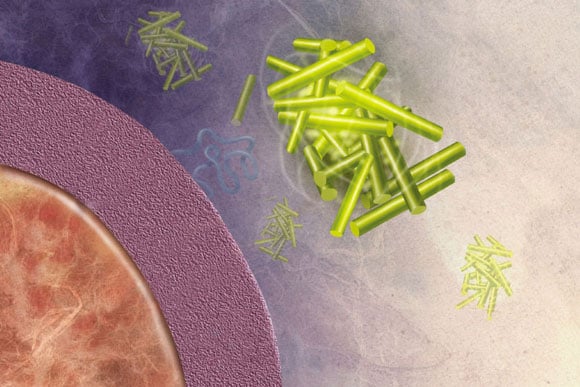
MIT biologists found that the yeast protein Rim4 forms disordered clumps similar to the beta-amyloid plaques associated with Alzheimer’s disease, shown here. Image: National Institute on Aging/NIH
In a newly published study, biologists from MIT detail how a yeast protein may lead to anti-amyloid therapeutic opportunities.
Fibrous protein clumps known as amyloids are most often associated with diseases such as Alzheimer’s disease, where they form characteristic plaques in the brain.
Scientists first described amyloids about 150 years ago; they have since been tagged as key players in Parkinson’s disease, Huntington’s disease, and rheumatoid arthritis, as well as Alzheimer’s. However, recent findings suggest that this class of proteins may also have critical biological functions in healthy cells.
In a study appearing in this week’s issue of Cell, MIT biologists have discovered that yeast cells need to build amyloid-like structures during the production of reproductive cells called spores. Learning more about how yeast build and then break down these protein structures could help scientists develop drugs that destroy disease-causing amyloids, the researchers say.
“Amyloids in the brain persist for decades. We just can’t get rid of them, yet yeast cells seem to have a mechanism for getting rid of them in 15 minutes,” says Luke Berchowitz, a postdoc at MIT’s Koch Institute for Integrative Cancer Research and the paper’s lead author. “If we can harness that mechanism, and really understand it, that could lead to anti-amyloid therapeutic opportunities.”
The paper’s senior author is Angelika Amon, the Kathleen and Curtis Marble Professor in Cancer Research and a member of the Koch Institute. Other authors are undergraduate Margaret Walker, postdocs Greg Kabachinski and Thomas Carlile, associate professor of biology Wendy Gilbert, and professor of biology Thomas Schwartz.
Reproductive role
Berchowitz and colleagues came across the yeast amyloid-forming protein known as Rim4 while investigating how sexual reproduction works in yeast. Rim4 is a protein containing long regions of disorder and stretches rich in the amino acid asparagine, which is a hallmark of a type of amyloid-forming proteins known as prions.
Berchowitz and Amon had previously discovered that Rim4 latches onto messenger RNA (mRNA) molecules, which carry genetic information to the cell’s protein-building machinery. In the new Cell paper, the researchers found that Rim4 uses amyloid-like clusters to prevent these mRNA molecules from being transcribed into proteins.
This process regulates the formation of spores — reproductive cells that are analogous to eggs and sperm, the researchers found.
Yeast usually reproduce asexually, through a process called budding, but under certain high-stress conditions, they can also undergo sexual reproduction through creation of spores that fuse to form new cells. The MIT team found that as yeast cells near completion of sexual reproduction, Rim4 amyloid-like clusters are broken down, releasing mRNA required for the cells to complete meiosis — the specialized type of cell division that produces spores.
“None of us anticipated that the way Rim4 actually works is by formation of these aggregates,” says Scott Keeney, a member of the Memorial Sloan Kettering Cancer Center, who was not involved in the research. “We’re used to thinking of these as toxic aggregates, so to demonstrate that they actually have a useful function in cells is intriguing.”
The researchers also found preliminary data suggesting that an amyloid protein known as DAZL plays the same role in sperm formation in mice; they believe that similar proteins are probably found in every organism that reproduces sexually, including humans.
Berchowitz says it is still unclear why cells rely on amyloid-forming proteins for this type of regulation, but one advantage amyloids offer is their ability to withstand the harsh environments in which sexual reproduction cells are formed. “Amyloids are stable and they’re able to sequester things,” he says. “They’re very tough guardians.”
“A great opportunity”
Previously, scientists have found a few other examples of amyloid-forming proteins that have critical roles in normal cell functions: In fruit flies, the persistence of memory can rely on the formation of amyloid-like structures in the brain, and amyloids are also involved in the formation of the skin pigment melanin in humans.
Learning more about how cells break down those amyloids could help scientists develop new drugs for diseases such as Alzheimer’s, Parkinson’s, Huntington’s, and rheumatoid arthritis. Berchowitz is now working on figuring out how yeast cells regulate the breakdown of Rim4 aggregates.
“It’s a great opportunity to study assembly, regulation, and function of amyloids in living cells,” he says. “It’s pretty exciting that we can form them rapidly, synchronously, and abundantly.”
Reference: “Regulated Formation of an Amyloid-like Translational Repressor Governs Gametogenesis” by Luke E. Berchowitz, Greg Kabachinski, Margaret R. Walker, Thomas M. Carlile, Wendy V. Gilbert, Thomas U. Schwartz and Angelika Amon, 24 September 2015, Cell.
DOI: 10.1016/j.cell.2015.08.060





Amyloid proteins in the brain exists from the beginning. But only the formation of plaques should be thwarted to avoid Alzheimer’s disease. Here the yeast cell requires amyloid plaques for formation of spores in their reproduction stage. They know the art of breaking the plaques later. Humans don’t have the instrument to break the plaques. But amyloid proteins are formed to probably strengthen the neural synopsis and network, and since they should not be rigid they undergo natural disintegration in the normal brain cells periodically. As the brain ages this disintegration of amyloid beta proteins which form plaques doesn’t happen and neural pathway is completely blocked by their rigidity, which causes Alzheimerism. The study through yeast may well give us an instrument or medicine to break the plaques and help patients. Thank you.
Amyloids are insoluble fibrous protein aggregates sharing specific structural traits. They are insoluble and arise from at least 18 inappropriately folded versions of proteins and polypeptides present naturally in the body. These misfolded structures alter their proper configuration such that they erroneously interact with one another or other cell components forming insoluble fibrils. They have been associated with the pathology of more than 20 serious human diseases in that abnormal accumulation of amyloid fibrils in organs may lead to amyloidosis, and may play a role in various neurodegenerative disorders. Scientists’ studying through yeast provides other researchers who focus on diseases like Alzheimerism inspiration.