New and more precise acidity measurements may help make PFAS easier to track.
Per- and polyfluoroalkyl substances (PFAS) are nicknamed “forever chemicals” in part because their acidity helps them linger in the environment.
Many of these toxic chemicals are strongly acidic, so they readily shed protons and take on a negative charge, which lets them dissolve in water and spread more easily.
New work shows some PFAS are even more acidic than earlier estimates — a key piece of information for forecasting their movement through the environment and their potential effects on human health.
The study, led by the University at Buffalo, introduced a rigorous experimental approach to determine acidity for 10 PFAS types and three common breakdown products.
Published in the journal Environmental Science & Technology Letters, the team reported acid dissociation constants, or pKa values, that were generally lower and sometimes far lower than prior experimental results and predictions from computational chemistry. For example, the pKa of GenX, a replacement for perfluorooctanoic acid (PFOA) used in Teflon manufacturing, was about one thousand times lower than a previously reported measurement.
The lower the pKa, the more likely a chemical is to give up a proton and exist in its charged form.
“These findings suggest that previous measurements have underestimated PFAS’ acidity. This means their ability to persist and spread in the environment has been mischaracterized, too,” says the study’s corresponding author, Alexander Hoepker, PhD, a senior research scientist with the UB RENEW Institute.
More accurate pKa measurements help efforts to understand the behavior of PFAS in the environment. A chemical’s pKa could mean the difference as to whether it remains dissolved in water, sticks to soil or a biological membrane, or perhaps volatilizes into the air.
“If we’re going to understand how these concerning chemicals spread, it’s very important we have a reliable method for the accurate determination of their pKa values,” says Diana Aga, PhD, director of RENEW and SUNY Distinguished Professor and Henry M. Woodburn Chair in the UB Department of Chemistry.
The work was supported by the National Science Foundation and done in collaboration with Scott Simpson, PhD, professor and chair of the St. Bonaventure Department of Chemistry, and researchers from Spain’s Institute of Environmental Assessment and Water Research.
Combining experiments with computations
PFAS are made of a highly fluorinated, water-repelling tail and a more water-loving headgroup. Many of the most scrutinized PFAS have a highly acidic headgroup, making them more likely to give up a proton and exist in its charged form.
Whether a PFAS exists in its neutral or charged form depends on the pH level of their surrounding environment. That’s where pKa comes in. It tells scientists the pH level at which a given PFAS is equal to flip from neutral to charged, or vice versa.
But there has been much disagreement about the pKa measurements of some PFAS, like PFOA, with different teams coming up with widely different values. One of the reasons for this may be the glass used during their experiments.
“PFAS likes to stick to glass. When that happens, it throws off traditional, so-called bulk measurements that quantify how much PFAS is in a solution,” Hoepker says. “In other cases, too much organic solvent is used to get PFAS into solution, which similarly biases the pKa measurement.”
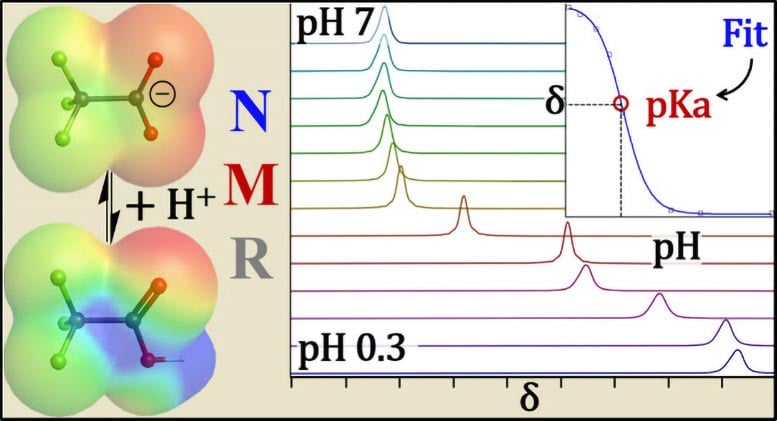
To address this challenge, the UB team used fluorine and proton (hydrogen) nuclear magnetic resonance (NMR) spectroscopy — think MRI for molecules. NMR places a sample in a strong magnetic field and probes its atomic nuclei with radio waves.
When a PFAS headgroup is negatively charged, nearby fluorine atoms respond at a different (radio) frequency.
Reading these atom-level signatures lets the researchers tell whether a PFAS molecule is charged or neutral — capabilities that other methods that have been used previously cannot provide.
“This unique measurement allows NMR to inherently account for PFAS losses to glass or other adsorption behaviors, so your pKa measurements don’t end up way off the mark,” Hoepker says.
Some PFAS are so acidic (pKa of less than zero) that generating them in their neutral form would require super-acidic conditions (a pH level of less than zero) that are impractical in standard labs. In those cases, the research team paired NMR experiments with electronic-structure calculations using density functional theory to predict the NMR shifts of the neutral and ionized forms.
“We augmented partial NMR datasets with computational predictions to arrive at more accurate pKa values,” Hoepker says. “This NMR-centered hybrid approach — integrating experimental measurements with computational analyses — enhanced our confidence in the results and, to our knowledge, has not previously been applied to PFAS acidity.”
Problem PFAS measured more accurately
The PFAS that has been the most difficult to measure is PFOA, once commonly used in nonstick pans and deemed hazardous by the Environmental Protection Agency last year.
The team found its pKa to be –0.27, meaning it will be negatively charged at practically any realistic pH level. Previous experimental studies had measured its pKa as high as 3.8 and more commonly around 1, while the computational methods COSMO-RS and OPERA had determined its pKa at 0.24 and 0.34, respectively.
Trifluoroacetic acid (TFA) — an emerging PFAS increasingly detected in waters worldwide and likely transported through the atmosphere and deposited by rain — was found to be far more acidic than previously reported, with a pKa of around 0.03. Earlier estimates had anywhere from 0.30 to 1.1.
Notably, the team determined the pKa values for several prominent emerging PFAS that had never been measured, such as 5:3 fluorotelomer carboxylic acid (5:3 FTCA), and PFAS ethers like NFDHA and PFMPA that are newer PFAS but are also likely to pose challenges for regulators due to their health effects.
“This new experimental approach of determining pKa values for PFAS will have wide-ranging applications, from being able to validate computationally derived values, to facilitating the development of machine learning models that can better predict pKa values of newly discovered PFAS contaminants when reference standards are not available,” Aga says. “In turn, knowledge of the pKa values of emerging PFAS will allow researchers to develop appropriate analytical methods, remediation technologies, and risk assessment strategies more efficiently.”
Reference: “Experimental Determination of pKa for 10 PFAS, Mono-, Di-, and Trifluoroacetic Acid by 19F-NMR” by Damalka Balasuryia, Aina Queral-Beltran, Tristan Vick, Scott Simpson, Silvia Lacorte, Diana S. Aga and Alexander C. Hoepker, 12 August 2025, Environmental Science & Technology Letters.
DOI: 10.1021/acs.estlett.5c00688
Funding: U.S. National Science Foundation
Never miss a breakthrough: Join the SciTechDaily newsletter.