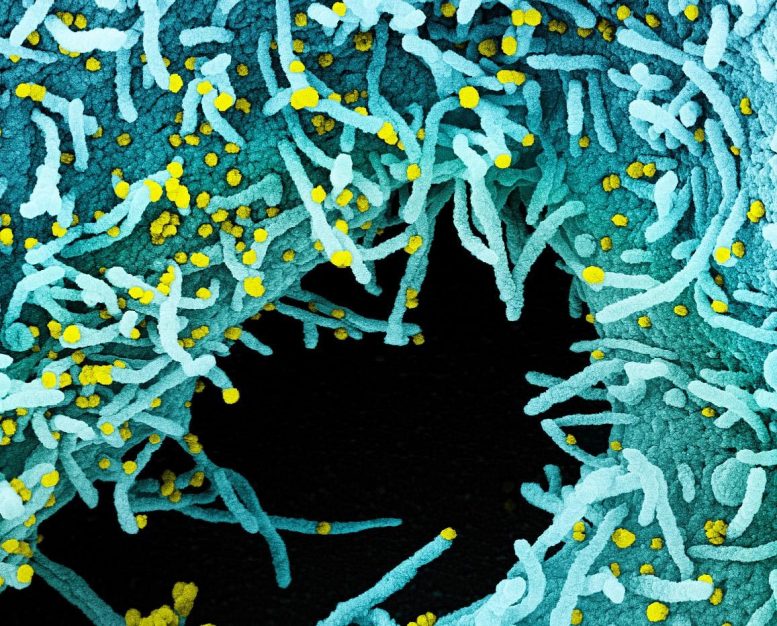
Colorized scanning electron micrograph of a cell heavily infected with SARS-CoV-2 virus particles (yellow), isolated from a patient sample. The black area in the image is extracellular space between the cells. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
NIAID-sponsored phase 1 trial tested mRNA vaccine
An investigational vaccine, mRNA-1273, designed to protect against SARS-CoV-2, the virus that causes coronavirus disease 2019 (COVID-19), was generally well tolerated and prompted neutralizing antibody activity in healthy adults, according to interim results published online today in The New England Journal of Medicine. The ongoing Phase 1 trial is supported by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. The experimental vaccine is being co-developed by researchers at NIAID and at Moderna, Inc. of Cambridge, Massachusetts. Manufactured by Moderna, mRNA-1273 is designed to induce neutralizing antibodies directed at a portion of the coronavirus “spike” protein, which the virus uses to bind to and enter human cells.
The trial was led by Lisa A. Jackson, M.D., MPH, of Kaiser Permanente Washington Health Research Institute in Seattle, where the first participant received the candidate vaccine on March 16. This interim report details the initial findings from the first 45 participants ages 18 to 55 years enrolled at the study sites in Seattle and at Emory University in Atlanta. Three groups of 15 participants received two intramuscular injections, 28 days apart, of either 25, 100, or 250 micrograms (mcg) of the investigational vaccine. All the participants received one injection; 42 received both scheduled injections.
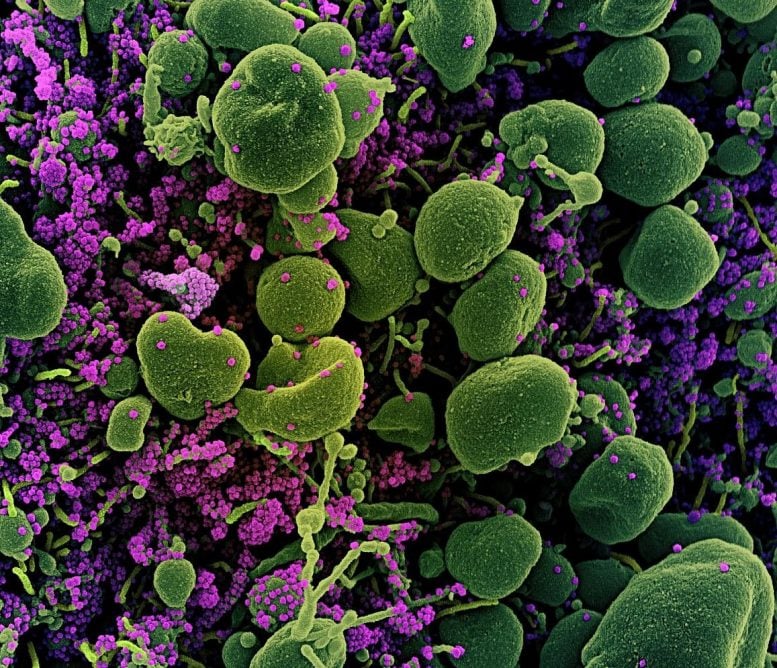
Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
In April, the trial was expanded to enroll adults older than age 55 years; it now has 120 participants. However, the newly published results cover the 18 to 55-year age group only.
Regarding safety, no serious adverse events were reported. More than half of the participants reported fatigue, headache, chills, myalgia, or pain at the injection site. Systemic adverse events were more common following the second vaccination and in those who received the highest vaccine dose. Data on side effects and immune responses at various vaccine dosages informed the doses used or planned for use in the Phase 2 and 3 clinical trials of the investigational vaccine.
The interim analysis includes results of tests measuring levels of vaccine-induced neutralizing activity through day 43 after the second injection. Two doses of vaccine prompted high levels of neutralizing antibody activity that were above the average values seen in convalescent sera obtained from persons with confirmed COVID-19 disease.
A Phase 2 clinical trial of mRNA-1273, sponsored by Moderna, began enrollment in late May. Plans are underway to launch a Phase 3 efficacy trial in July 2020.
Reference: “An mRNA Vaccine against SARS-CoV-2 — Preliminary Report” by Lisa A. Jackson, M.D., M.P.H., Evan J. Anderson, M.D., Nadine G. Rouphael, M.D., Paul C. Roberts, Ph.D., Mamodikoe Makhene, M.D., M.P.H., Rhea N. Coler, Ph.D., Michele P. McCullough, M.P.H., James D. Chappell, M.D., Ph.D., Mark R. Denison, M.D., Laura J. Stevens, M.S., Andrea J. Pruijssers, Ph.D., Adrian McDermott, Ph.D., Britta Flach, Ph.D., Nicole A. Doria-Rose, Ph.D., Kizzmekia S. Corbett, Ph.D., Kaitlyn M. Morabito, Ph.D., Sijy O’Dell, M.S., Stephen D. Schmidt, B.S., Phillip A. Swanson, II, Ph.D., Marcelino Padilla, B.S., John R. Mascola, M.D., Kathleen M. Neuzil, M.D., Hamilton Bennett, M.Sc., Wellington Sun, M.D., Etza Peters, R.N., Mat Makowski, Ph.D., Jim Albert, M.S., Kaitlyn Cross, M.S., Wendy Buchanan, B.S.N., M.S., Rhonda Pikaart-Tautges, B.S., Julie E. Ledgerwood, D.O., Barney S. Graham, M.D. and John H. Beigel, M.D. for the mRNA-1273 Study Group, 14 July 2020, New England Journal of Medicine.
DOI: 10.1056/NEJMoa2022483
Additional information about the Phase 1 clinical trial design is available at clinicaltrials.gov using the identifier NCT04283461. This trial was supported in part by the NIAID grants M1AI148373 (Kaiser Permanente Washington), UM1AI148576 (Emory University) and UM1AI148684 (Infectious Diseases Clinical Research Consortium). Funding for the manufacture of mRNA-1273 Phase 1 material was provided by the Coalition for Epidemic Preparedness Innovations (CEPI).





Be the first to comment on "COVID-19 Vaccine Phase 1 Trial Results: Safe, Generates High Levels of Neutralizing Antibodies"