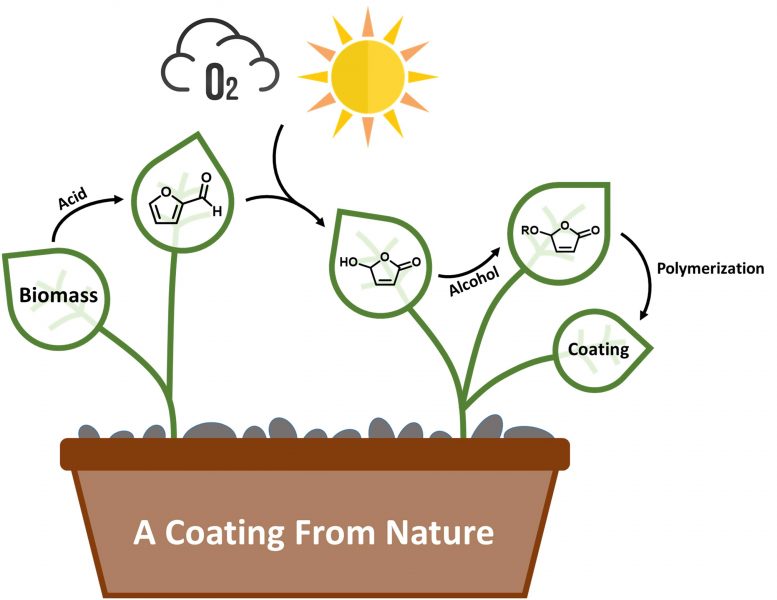
Lignocellulose biomass is cracked using acid to produce furfural. Using visible light and oxygen, furfural is converted into hydroxybutenolide, which is then modified using different alcohols to produce alkoxybutenolide monomers, that can be polymerized into coatings using UV light. Credit: George Hermens and Paco Visser, University of Groningen
Organic chemists from the University of Groningen and the Dutch multinational company AkzoNobel, a major global producer of paints and coatings, developed a process that allows them to turn biomass into a high-quality coating using light, oxygen, and UV light. This process combines a renewable source with green chemistry and could replace petrochemical-based monomers such as acrylates, which are currently used as building blocks for coatings, resins, and paints. A paper on the new process was published in the journal Science Advances on December 16, 2020.
Coatings are everywhere, from the paint on your house to a protective layer on the screen of your smartphone. They protect surfaces from scratches, influences of the weather, or everyday wear. Most coatings are made up of polymers based on acrylate monomers, with the global production of acrylate exceeding 3.5 million tonnes a year, all produced from fossil oil.
Biomass
To make these coatings more sustainable, scientists from the University of Groningen, led by Professor of Organic Chemistry Ben Feringa, teamed up with scientists from coating producer AkzoNobel. “We wanted to use lignocellulose as the starting material,” says George Hermens, a PhD student in the Feringa group and first author of the paper in Science Advances. Lignocellulose makes up 20 to 30 percent of the woody parts of plants and is the most abundantly available raw biomass material on Earth. Currently, it is mainly used as a solid fuel or used to produce biofuels.
This is University of Groningen Professor of Organic Chemistry Ben Feringa, co-laureate of the 2016 Nobel Prize for Chemistry. who lead the project to create coatings from nature. Credit: Jeroen van Kooten
“Lignocellulose can be cracked with acid to produce the chemical building block furfural, but this needs to be modified to make it suitable for the production of coatings,” explains Hermens. He used a process that has been developed in their group to convert the furfural into a compound, hydroxybutenolide, that resembles acrylic acid. “The chemical conversion uses only light, oxygen, and a simple catalyst and produces no waste. The only side product is methyl formate, which is useful as a replacement for chlorofluorocarbons in other processes.”
Properties
Part of the structure of hydroxybutenolide is similar to acrylate, but the reactive part of the molecule is a ring structure. “This means that it is less reactive than acrylate and our challenge was to further modify the molecule so that it would produce a useful polymer.” This was achieved by adding different green or biobased alcohols to the hydroxybutenolide, creating four different alkoxybutenolide monomers.

This picture shows first and second authors of the paper, Thomas Freese (left) and George Hermens, in front of the flow system used for the conversion of furfural into hydroxybutenolide. Credit: Feringa Lab, University of Groningen
These monomers can be transformed into polymers and coatings with the help of an initiator and UV light. “Coatings are made up of cross-linked polymer chains. By combining different monomers, we could get cross-linked polymers with different properties.” For example, while all polymers would coat glass, one combination was able to also form a coating on plastic. And by adding more rigid monomers, a harder coating was formed, with properties comparable to those of coatings on cars. In this way, these coatings are adaptable for different purposes.
Product development
“We managed to create coatings from a renewable source, lignocellulose, using green chemistry,” concludes Hermens. “And the quality of our coatings is similar to that of current acrylate-based coatings.” For two steps in the process, patent applications have been filed with AkzoNobel, the industrial partner in the project. Hermens is now working on a different building block derived from furfural to produce other types of polymer coatings.
The project was initiated by the Advanced Research Center Chemical Building Blocks Consortium (ARC CBBC), a Dutch national public-private research center that develops new chemical processes and chemical building blocks for novel energy carriers, materials, and chemicals for sustainable chemistry. Hermens’ supervisor, Ben Feringa, is one of the founders of this center. The ARC CBBC is a national initiative with partners from industry, academia, and government. There are three universities involved (Utrecht University, the University of Groningen, and Eindhoven University of Technology) and major industrial partners (AkzoNobel, Shell, Nouryon, and BASF), as well as the ministries of Education, Culture and Science and Economic Affairs and Climate Policy and the Dutch Research Council (NWO). Feringa: “The program entails all the steps from fundamental scientific discovery to process and product development. In this long-term partnership, universities and the chemical industry join forces to develop the green chemistry of the future.”
Reference: “A coating from nature” by Johannes G. H. Hermens, Thomas Freese, Keimpe J. van den Berg, Rogier van Gemert and Ben L. Feringa, 16 December 2020, Science Advances.
DOI: 10.1126/sciadv.abe0026







Be the first to comment on "Green Chemistry Creates Coatings From Nature: Turning Biomass Into High-Quality Coatings"