
Researchers created a stable sodium-ion conductor for solid-state batteries, enhancing the efficiency and lifespan of batteries using higher-voltage oxide cathodes.
A team of researchers designed and manufactured a new sodium-ion conductor for solid-state sodium-ion batteries that is stable when incorporated into higher-voltage oxide cathodes. This new solid electrolyte could dramatically improve the efficiency and lifespan of this class of batteries. A proof-of-concept battery built with the new material lasted over 1000 cycles while retaining 89.3% of its capacity—a performance unmatched by other solid-state sodium batteries to date.
Researchers detail their findings in the February 23, 2021 issue of Nature Communications.
Solid state batteries hold the promise of safer, cheaper, and longer lasting batteries. Sodium-ion chemistries are particularly promising because sodium is low-cost and abundant, as opposed to the lithium required for lithium-ion batteries, which is mined at a high environmental cost. The goal is to build batteries that can be used in large-scale grid energy storage applications, especially to store power generated by renewable energy sources to mitigate peak demand.
“Industry wants batteries at cell-level to cost $30 to $50 per kWh,” about one-third to one-fifth of what it costs today, said Shirley Meng, a professor of nanoengineering at the University of California San Diego, and one of the paper’s corresponding authors. ‘We will not stop until we get there.”
The ZrCl6 unit is shown here rotating, creating vacancies, which increases conductivity. Credit: University of California
The work is a collaboration between researchers at UC San Diego and UC Santa Barbara, Stony Brook University, the TCG Center for Research and Education in Science and Technology in Kolkata, India, and Shell International Exploration, Inc.
For the battery described in the Nature Communications study, researchers led by UC San Diego nanoengineering professor Shyue Ping Ong ran a series of computational simulations powered by a machine learning model to screen which chemistry would have the right combination of properties for a solid state battery with an oxide cathode. Once a material was selected as a good candidate, Meng’s research group experimentally fabricated, tested, and characterized it to determine its electrochemical properties.
By rapidly iterating between computation and experiment, the UC SanDiego team settled on a class of halide sodium conductors made up of sodium, yttrium, zirconium, and chloride. The material, which they named NYZC, was both electrochemically stable and chemically compatible with the oxide cathodes used in higher voltage sodium-ion batteries. The team then reached out to researchers at UC Santa Barbara to study and understand the structural properties and behavior of this new material.
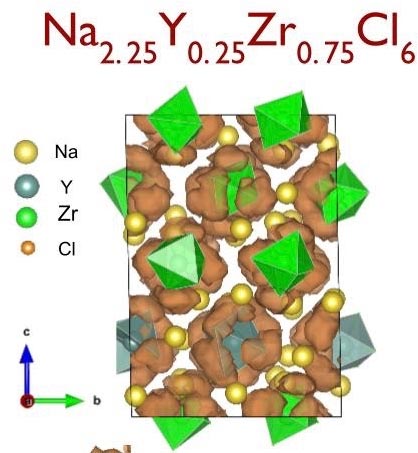
If Zr-Cl rotation is artificially frozen, sodium diffusivity plummets to negligible results. Zr-Cl rotation thus aids sodium conductivity. Credit: University of California San Diego
NYZC is based on Na3YCl6, a well-known material that is unfortunately a very poor sodium conductor. Ong suggested substituting zirconium for yttrium because it would create vacancies and increase the volume of the cell battery unit, two approaches that increase the conduction of sodium ions. Researchers also noted that, in conjunction with the increased volume, a combination of zirconium and chloride ions in this new material undergoes a rotating motion, resulting in more conduction pathways for the sodium ions. In addition to the increase in conductivity, the halide material is much more stable than materials currently used in solid-state sodium batteries.
“These findings highlight the immense potential of halide ion conductors for solid-state sodium-ion battery applications,” said Ong. “Further, it also highlights the transformative impact that large-scale materials data computations coupled with machine learning can have on the materials discovery process.”
Next steps include exploring other substitutions for these halide materials and increasing the battery’s overall power density, along with working to scale up the manufacturing process.
Reference: “A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries” by Erik A. Wu, Swastika Banerjee, Hanmei Tang, Peter M. Richardson, Jean-Marie Doux, Ji Qi, Zhuoying Zhu, Antonin Grenier, Yixuan Li, Enyue Zhao, Grayson Deysher, Elias Sebti, Han Nguyen, Ryan Stephens, Guy Verbist, Karena W. Chapman, Raphaële J. Clément, Abhik Banerjee, Ying Shirley Meng and Shyue Ping Ong, 23 February 2021, Nature Communications.
DOI: 10.1038/s41467-021-21488-7
The technology has been licensed by UNIGRID, a startup co-founded by UC San Diego NanoEngineering professor Zheng Chen; Erik Wu, a Ph.D. alumnus from Meng’s research group; and Darren H. S. Tan, one of Meng’s Ph.D. students. Meng is the company’s technical advisor.
Funding to support this work was provided by the Energy & Biosciences Institute through the EBI-Shell program and NSF.








While the concept of a cheaper sodium-ion battery is exciting, from the data given in Figure 5 of the Nature article, the authors’ sodium-ion battery does not work at low temperatures. At 20 °C (68 °F) and only half the rated charge rate (C/2), the capacity and cycle life are both reduced by more than a half. Any lower temperatures and/or higher charge rates would be detrimental to the cells. Moreover, even at elevated temperatures, the specific power is no higher than that of an industry-standard lithium-ion battery. Therefore, the lack of the ability to work at low temperatures and/or high charge rates will probably preclude the use of the proposed solid-state sodium-ion battery in automotive applications.
To whom it may concern: I can provide Sodium for the solid state battery. Please send me your inquiry to my email at [email protected] Best Regards, Boon Chompoonich
Dear Mr Swastika Banerjee and other authors,
The sodium and clorine sounds good, I know exactly where we can source giga tons of that for a great price.
But the yttrium will be $2700/kg and zirconium $150/kg might be a problem, could the team please find cheaper alternates. So how much is needed per KWhr of storage materials since you are intending to make a $30KWhr cell?
Sounds like 10g of yttrium at $27 has already blown the target cost, are you using only micro grams per cell?
sincerely
Infinite energy materials corp
Okay from the eqn it looks like 9 parts sodium, 1 part yttrium plus the other 2 elements, that is way more yttrium than I can source from China. So $3 worth of sodium mixes with $300 worth of yttrium plus what ever. So really this is a yttrium battery is it not?
Clarify yttrium is not quite a rare earth, but is found mostly with the lanthanides.
23-Sodium at $3/kg vs 89-Yttrium at $2700/kg so really 900x the price by kg, but 3600x more by mole. So this really is a yttrium battery on cost basis.
There is a great US source of lanthanides that would produce copius amounts of thorium at about $30/kg and probably some yttrium too. While thorium isn’t going to be useful in a rechargeable battery, it could be used in a nuclear reactor giving about 1000kWyr or 9M kWhr of energy per kg. That embedded energy value is worth $1M from just $30 of metal for 11c/kWhr.
It’s hard to say how much renewable energy $30 worth of yttrium could help store, but I doubt it would be anything like 9M kWhr over battery life.
I am not a chemist and I don’t understand why in this list “… sodium, yttrium, zirconium and chloride….” three of the items are elements and one is not ie why isn’t the list “… sodium, yttrium, zirconium and chlorone”?
Amazing that a non chemist can spot a bug in the magical writings of so many Phd chemists, but you also meant chlorine, but they almost certainly made a typo too, minor mistake.
The bigger problem is the yttrium problem.