
In crisis, the nucleus calls antioxidant enzymes to the rescue. The nucleus being metabolically active is a profound paradigm shift with implications for cancer research.
- The human nucleus is metabolically active, according to the findings of a new study in Molecular Systems Biology by researchers at the CRG in Barcelona and CeMM/Medical University of Vienna.
- In a state of crisis, such as widespread DNA damage, the nucleus protects itself by appropriating mitochondrial machinery to carry out urgent repairs that threaten the genome’s integrity.
- The findings represent a paradigm shift because the nucleus has been historically considered to be metabolically inert, importing all its needs through supply chains in the cytoplasm.
- Cancer hijacks cellular metabolism for unfettered growth. The findings can help guide future lines of cancer research by offering new clues to overcome drug resistance and eventually the design of new treatments.
The Double-Edged Sword of Cellular Metabolism
A typical human cell is metabolically active, roaring with chemical reactions that convert nutrients into energy and useful products that sustain life. These reactions also create reactive oxygen species, dangerous by-products like hydrogen peroxide which damage the building blocks of DNA in the same way oxygen and water corrode metal and form rust. Just how buildings collapse from the cumulative effect of rust, reactive oxygen species threaten a genome’s integrity.
Cells are thought to delicately balance their energy needs and avoid damaging DNA by containing metabolic activity outside the nucleus and within the cytoplasm and mitochondria. Antioxidant enzymes are deployed to mop up reactive oxygen species at their source before they reach DNA, a defensive strategy that protects the roughly 3 billion nucleotides from suffering potentially catastrophic mutations. If DNA damage occurs anyway, cells pause momentarily and carry out repairs, synthesizing new building blocks and filling in the gaps.
Despite the central role of cellular metabolism in maintaining genome integrity, there has been no systematic, unbiased study on how metabolic perturbations affect the DNA damage and repair process. This is particularly important for diseases like cancer, characterized by their ability to hijack metabolic processes for unfettered growth.

A research team led by Sara Sdelci at the Centre for Genomic Regulation (CRG) in Barcelona and Joanna Loizou at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences in Vienna and the Medical University of Vienna addressed this challenge by carrying out various experiments to identify which metabolic enzymes and processes are essential for a cell’s DNA damage response. The findings are published today in the journal Molecular Systems Biology.
A Paradigm Shift: The Nucleus as a Metabolic Hub
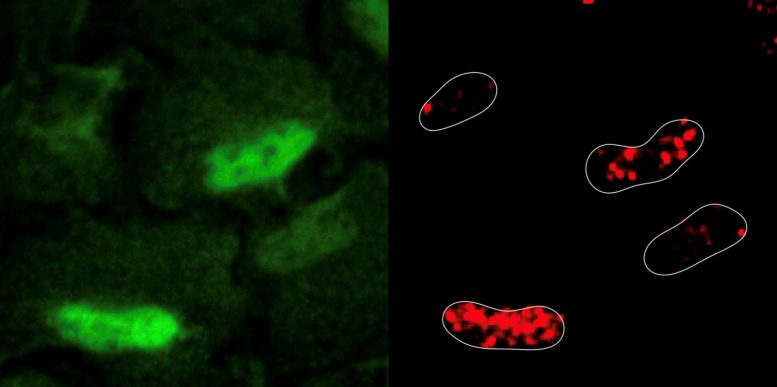
The researchers experimentally induced DNA damage in human cell lines using a common chemotherapy medication known as etoposide. Etoposide works by breaking DNA strands and blocking an enzyme that helps repair the damage. Surprisingly, inducing DNA damage resulted in reactive oxygen species being generated and accumulating inside the nucleus. The researchers observed that cellular respiratory enzymes, a major source of reactive oxygen species, relocated from the mitochondria to the nucleus in response to DNA damage.
The findings represent a paradigm shift in cellular biology because it suggests the nucleus is metabolically active. “Where there’s smoke there’s fire, and where there are reactive oxygen species there are metabolic enzymes at work. Historically, we’ve thought of the nucleus as a metabolically inert organelle that imports all its needs from the cytoplasm, but our study demonstrates that another type of metabolism exists in cells and is found in the nucleus,” says Dr. Sara Sdelci, corresponding author of the study and Group Leader at the Centre for Genomic Regulation.
The researchers also used CRISPR-Cas9 to identify all the metabolic genes that were important for cell survival in this scenario. These experiments revealed that cells order the enzyme PRDX1, an antioxidant enzyme also normally found in mitochondria, to travel to the nucleus and scavenge reactive oxygen species present to prevent further damage. PRDX1 was also found to repair the damage by regulating the cellular availability of aspartate, a raw material that is critical for synthesizing nucleotides, the building blocks of DNA.
“PRDX1 is like a robotic pool cleaner. Cells are known to use it to keep their insides “clean” and prevent the accumulation of reactive oxygen species, but never before at the nuclear level. This is evidence that, in a state of crisis, the nucleus responds by appropriating mitochondrial machinery and establishes an emergency rapid-industrialization policy,” says Dr. Sdelci.
The findings can guide future lines of cancer research. Some anti-cancer drugs, such as etoposide used in this study, kill tumor cells by damaging their DNA and inhibiting the repair process. If enough damage accumulates, the cancer cell initiates a process where it autodestructs.
During their experiments, the researchers found that knocking out metabolic genes critical for cellular respiration – the process that generates energy from oxygen and nutrients – made normal healthy cells become resistant to etoposide. The finding is important because many cancer cells are glycolytic, meaning that even in the presence of oxygen they generate energy without doing cellular respiration. This means etoposide, and other chemotherapies with a similar mechanism, are likely to have a limited effect in treating glycolytic tumors.
Potential New Strategies for Cancer Therapy
The authors of the study call for the exploration of new strategies such as dual treatment combining etoposide with drugs that also boost the generation of reactive oxygen species to overcome drug resistance and kill cancer cells faster. They also hypothesize that combining etoposide with inhibitors of nucleotide synthesis processes could potentiate the effect of the drug by preventing the repair of DNA damage and ensuring cancer cells self-destruct correctly.
Dr. Joanna Loizou, corresponding author and Group Leader at the Center for Molecular Medicine and the Medical University of Vienna, highlights the value of taking data-driven approaches to uncover new biological processes. “By using unbiased technologies such as CRISPR-Cas9 screening and metabolomics, we have learned about how the two fundamental cellular processes of DNA repair and metabolism are intertwined. Our findings shed light on how targeting these two pathways in cancer might improve therapeutic outcomes for patients.”
Reference: “A metabolic map of the DNA damage response identifies PRDX1 in the control of nuclear ROS scavenging and aspartate availability” by Amandine Moretton, Savvas Kourtis, Antoni Gañez Zapater, Chiara Calabrò, Maria Lorena Espinar Calvo, Frédéric Fontaine, Evangelia Darai, Etna Abad Cortel, Samuel Block, Laura Pascual-Reguant, Natalia Pardo-Lorente, Ritobrata Ghose, Matthew G Vander Heiden, Ana Janic, André C Müller, Joanna I Loizou and Sara Sdelci, 1 June 2023, Molecular Systems Biology.
DOI: 10.15252/msb.202211267
Never miss a breakthrough: Join the SciTechDaily newsletter.
4 Comments
Muy bueno. I hope their discoveries lead to new, improved treatments for Cancer; perhaps even a cure at last.
Is it possible, those w/strong cancer history have an insufficient response with PRDX1?
I wish you success in doing this anti cancer research. Thanks.
I think there is a chance at immortality!