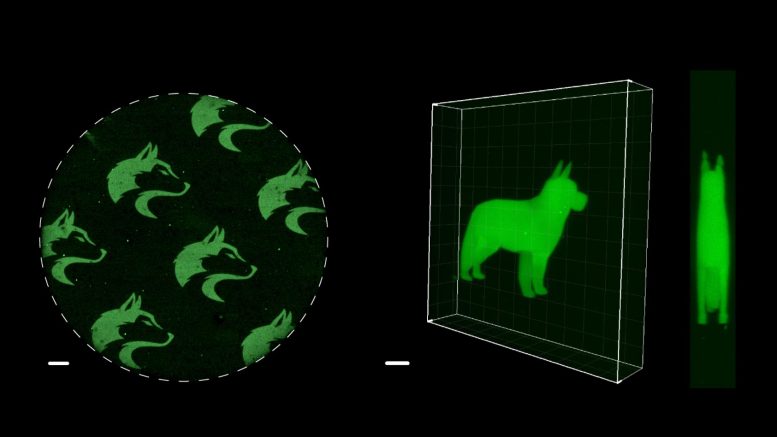
Microscopic 2D and 3D images of University of Washington Husky logos and a dog were made with a new chemistry technique, SpyLigation, that precisely controls when and where proteins turn on. Credit: Cole DeForest Research Group
This light-activation technology has potential applications in tissue engineering, regenerative medicine, and understanding how the body works.
Scientists have developed light-activated SpyLigation, a method using light to activate protein functions in and outside living cells. This has potential applications in tissue engineering, regenerative medicine, and understanding bodily processes. The technique involves joining chemically modified protein fragments with light and has been demonstrated in controlling protein glow and activating proteins inside human cells.
Scientists can now use light to activate protein functions both inside and outside of living cells. The new method, called light-activated SpyLigation, can turn on proteins that are normally off to allow researchers to study and control them in more detail. This technology has potential uses in tissue engineering, regenerative medicine, and understanding how the body works.
Proteins perform nearly every important task in biology, including processing DNA, metabolizing nutrients, and fighting off infections. When, where, and how proteins become active is important for a variety of biological processes. Increasingly, scientists are also exploring whether protein functions can be turned on and off to treat disease.
“With new tools for controlling protein function, particularly those that offer controlled activation in time and space, we are working towards engineering complex tissue for transplantation,” said senior author Cole A. DeForest, a Weyerhaeuser Endowed Associate Professor of Chemical Engineering at the University of Washington College of Engineering and an associate professor of bioengineering, a joint department at the UW College of Engineering and School of Medicine.
“Since many more people could benefit from tissue or organ transplants than there are available donors,” he said, “these methods offer real promise in combating the organ shortage crisis.”
As reported April 17 in the journal Nature Chemistry, a team led by Emily Ruskowitz and Brizzia Munoz-Robles from the DeForest Research Group has shown that chemically modified protein fragments can be joined together into functional wholes using brief flashes of light.
The scientists applied their new method to control the glow of a green fluorescent protein derived from Japanese eel muscle. Inactive fragments of that protein were blended and set into a Jell-O-like gel. Then lasers were used to irreversibly recombine those fragments into complete, glowing proteins. By controlling the path of the laser, a precise pattern of glowing proteins could be formed. The scientists etched microscopic images of a husky, their university mascot, into the gel. They also used lasers to create a glowing 3D image of a dog not much taller than a human hair.
The team also showed they could activate proteins inside human cells. Three minutes of light exposure was enough to turn on specific proteins involved in genome editing. Such a tool could one day be used to direct genetic changes to very specific areas of the body.
Similar to so-called click chemistry, which was the subject of the 2022 Nobel Prize in Chemistry, light-activated SpyLigation allows modified proteins to react with one another inside living systems. Extending beyond prior approaches, however, the new method allows for precise control over when and where such chemical reactions occur.
Reference: “Spatiotemporal Functional Assembly of Split Protein Pairs through a Light-Activated SpyLigation” by Emily R. Ruskowitz, Brizzia G. Munoz-Robles, Alder C. Strange, Carson H. Butcher, Sebastian Kurniawan, Jeremy R. Filteau and Cole A. DeForest, 17 April 2023, Nature Chemistry.
DOI: 10.1038/s41557-023-01152-x
This work was supported by a CAREER Award (DMR 1652141) and grants (DMR 1807398, CBET 1803054), from the National Science Foundation, as well as a Maximizing Investigators’ Research Award (R35GM138036) from the National Institutes of Health. Student fellowship support was provided by the Institute for Stem Cells & Regenerative Medicine, and the Mary Gates Endowment for Students at the University of Washington. Part of this work was conducted with instrumentation provided by the Joint Center for Deployment and Research in Earth Abundant Materials. The Thorlabs multiphoton microscope was acquired with and operated under support from the Washington Research Foundation, UW College of Engineering, UW Medicine Institute for Stem Cells and Regenerative Medicine, and the UW chemical engineering, bioengineering, chemistry, and biology departments.
DeForest is an investigator at UW Medicine’s Institute for Protein Design and Institute for Stem Cell and Regenerative Medicine, and the UW Molecular Engineering & Science Institute.







Be the first to comment on "Protein Power Play: SpyLigation Flips the Switch With a Flash of Light"