Scientists developed a low-cost device that uses solar energy to split water and produce oxygen, moving a step closer to sustainable hydrogen fuel.
For decades, researchers around the world have searched for ways to use solar power to generate the key reaction for producing hydrogen as a clean energy source — splitting water molecules to form hydrogen and oxygen. However, such efforts have mostly failed because doing it well was too costly, and trying to do it at a low cost led to poor performance.
Now, researchers from The University of Texas at Austin have found a low-cost way to solve one-half of the equation, using sunlight to efficiently split off oxygen molecules from water. The finding, published recently in Nature Communications, represents a step forward toward greater adoption of hydrogen as a key part of our energy infrastructure.
As early as the 1970s, researchers were investigating the possibility of using solar energy to generate hydrogen. But the inability to find materials with the combination of properties needed for a device that can perform the key chemical reactions efficiently has kept it from becoming a mainstream method.
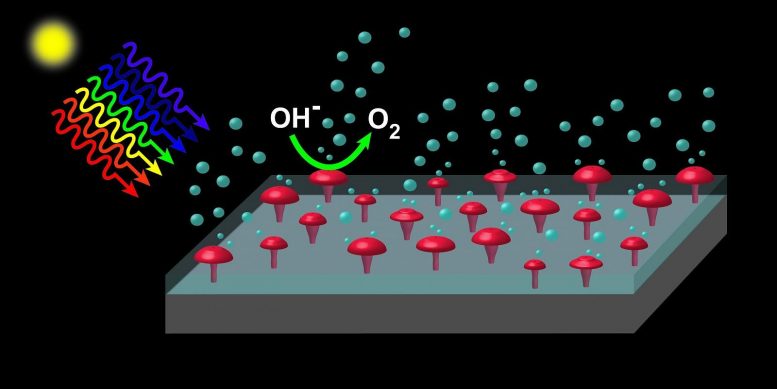
“You need materials that are good at absorbing sunlight and, at the same time, don’t degrade while the water-splitting reactions take place,” said Edward Yu, a professor in the Cockrell School’s Department of Electrical and Computer Engineering. “It turns out materials that are good at absorbing sunlight tend to be unstable under the conditions required for the water-splitting reaction, while the materials that are stable tend to be poor absorbers of sunlight. These conflicting requirements drive you toward a seemingly inevitable tradeoff, but by combining multiple materials — one that efficiently absorbs sunlight, such as silicon, and another that provides good stability, such as silicon dioxide — into a single device, this conflict can be resolved.”
However, this creates another challenge — the electrons and holes created by absorption of sunlight in silicon must be able to move easily across the silicon dioxide layer. This usually requires the silicon dioxide layer to be no more than a few nanometers, which reduces its effectiveness in protecting the silicon absorber from degradation.
The key to this breakthrough came through a method of creating electrically conductive paths through a thick silicon dioxide layer that can be performed at low cost and scaled to high manufacturing volumes. To get there, Yu and his team used a technique first deployed in the manufacturing of semiconductor electronic chips. By coating the silicon dioxide layer with a thin film of aluminum and then heating the entire structure, arrays of nanoscale “spikes” of aluminum that completely bridge the silicon dioxide layer are formed. These can then easily be replaced by nickel or other materials that help catalyze the water-splitting reactions.
Efficient, Scalable Oxygen Splitting Device
When illuminated by sunlight, the devices can efficiently oxidize water to form oxygen molecules while also generating hydrogen at a separate electrode and exhibit outstanding stability under extended operation. Because the techniques employed to create these devices are commonly used in manufacturing of semiconductor electronics, they should be easy to scale for mass production.
The team has filed a provisional patent application to commercialize the technology.
Improving the way hydrogen is generated is key to its emergence as a viable fuel source. Most hydrogen production today occurs through heating steam and methane, but that relies heavily on fossil fuels and produces carbon emissions.
There is a push toward “green hydrogen” which uses more environmentally friendly methods to generate hydrogen. Simplifying the water-splitting reaction is a key part of that effort.
Broader Implications for Energy and Industry
Hydrogen has the potential to become an important renewable resource with some unique qualities. It already has a major role in significant industrial processes, and it is starting to show up in the automotive industry. Fuel cell batteries look promising in long-haul trucking, and hydrogen technology could be a boon to energy storage, with the ability to store excess wind and solar energy produced when conditions are ripe for them.
Going forward, the team will work to improve the efficiency of the oxygen portion of water-splitting by increasing the reaction rate. The researchers’ next major challenge is then to move on to the other half of the equation.
“We were able to address the oxygen side of the reaction first, which is the more challenging part, ” Yu said, “but you need to perform both the hydrogen and oxygen evolution reactions to completely split the water molecules, so that’s why our next step is to look at applying these ideas to make devices for the hydrogen portion of the reaction.”
Reference: “Scalable, highly stable Si-based metal-insulator-semiconductor photoanodes for water oxidation fabricated using thin-film reactions and electrodeposition” by Soonil Lee, Li Ji, Alex C. De Palma and Edward T. Yu, 25 June 2021, Nature Communications.
DOI: 10.1038/s41467-021-24229-y
This research was funded by the U.S. National Science Foundation through the Directorate for Engineering and the Materials Research Science and Engineering Centers (MRSEC) program. Yu worked on the project with UT Austin students Soonil Lee and Alex De Palma, along with Li Ji, a professor at Fudan University in China.
Never miss a breakthrough: Join the SciTechDaily newsletter.
12 Comments
Electrolysis does not “oxidize water” so much as it “reduces hydrogen” by freeing it from oxygen. Reduction is the Red in RedOx reactions.
Well, journalists have never been known for understanding much about anything, especially anything technical.
There’s no such thing as green energy. Even solar takes light and heat energy which would have been used by various organisms in the vacinity.
Stars burn through enormous amounts of fuel and expand to destroy planets at a minimum and detonate to destroy everything within dozens of light years and leave a trash pile so toxic it kills physics at the max. Calm down with the preaching, we know.
Oh my. Ties with china so that evil Zi & CCP will have all the IP.
Deport any Chinese citizens with suspected ties to any military company there and jail any proven spy. These measures have been thrawted by Joe Xi-den’s DOJ who are going after perceived domestic terrorists instead.
That reply really needs follow-up with a health screening and I’m not pulling snark with this statement.
As much as neil enjoys TDS.
Frankie is absolutely correct.
Instead of name calling and diagnosing people based on your perception, prove him wrong.
I will wait.
You all are proving that in practically any news comments section it only takes two posts before descending into bicker. Well done lads. Tell me about your childhood…
The easiest way to split water is to use heat rather than electricity since it generally has about 1/3 the cost per Joule. The Sulfur Iodine catalytic process is 50% efficient when heat is available at around 700c.
To produce 1GW of continous hydrogen, it would take a 2GW thermal molten salt reactor at a cost near $1B plus the S-I plant. By contrasts it would take atleast baseload 1.5GWe supply which if solar would be 10GW of nameplate plus a whole lot of platinum based catalyzers at a cost closer to $100B.
If you can figure the cost of a moderate 10KWe nameplate solar system with battery system to make baseload 1.5KWe plus a 1KW catalyzer, I’d be surprised if the cost is less than $100K. Then multiply by 1M to get 1GW of hydrogen that would be closer to the scale of an oil refinery.
Every time I check google for solar system prices I always get prices an order of magnitude or more greater than what the solar fans would have us believe.
Would platinum still serve as a catalyst in these types of reactions? What does this mean for the platinum mining industry, anyone?
Hydrogen is the real clean sustainable energy source of the future Check out BrilliantlightPower(dot)com
Hydrogen is the clean sustainable energy source of the future out BrilliantlightPower(dot)com. Would love to see SciTechDaily interview the many scientists who have validated his work and discuss it! But would really love to See Silicon Valley-type money flow to his company to get it done and shipped!!! Change the world a lot faster!