Washington University’s novel antibiotic clears severe infections in mice and shows promise against drug-resistant bacteria. The compound speeds up healing and may soon enter clinical trials, aiming to provide a new solution in the fight against bacterial diseases.
A new antibiotic compound developed by Washington University researchers effectively treats severe bacterial infections, including flesh-eating disease, in mice.
The study, indicating broad-spectrum activity against drug-resistant bacteria, shows the compound also aids in quicker recovery and reduced infection severity. However, extensive testing and clinical trials are required before it reaches the market.
Breakthrough in Antibiotic Research
Researchers at Washington University School of Medicine in St. Louis have developed a novel compound that effectively clears bacterial infections in mice, including those that can result in rare but potentially fatal “flesh-eating” illnesses. The compound could be the first of an entirely new class of antibiotics, and a gift to clinicians seeking more effective treatments against bacteria that can’t be tamed easily with current antibiotics.
The research is published today (August 2) in the journal Science Advances.
The compound targets gram-positive bacteria, which can cause drug-resistant staph infections, toxic shock syndrome, and other illnesses that can turn deadly. It was developed through a collaboration between the labs of Scott Hultgren, PhD, the Helen L. Stoever Professor of Molecular Microbiology, and Michael Caparon, PhD, a professor of molecular microbiology, and Fredrik Almqvist, a professor of chemistry at the University of Umeå in Sweden.
A new type of antimicrobial would be good news for clinicians seeking effective treatments against pathogens that are becoming more resistant to currently available drugs, and thus much more dangerous.
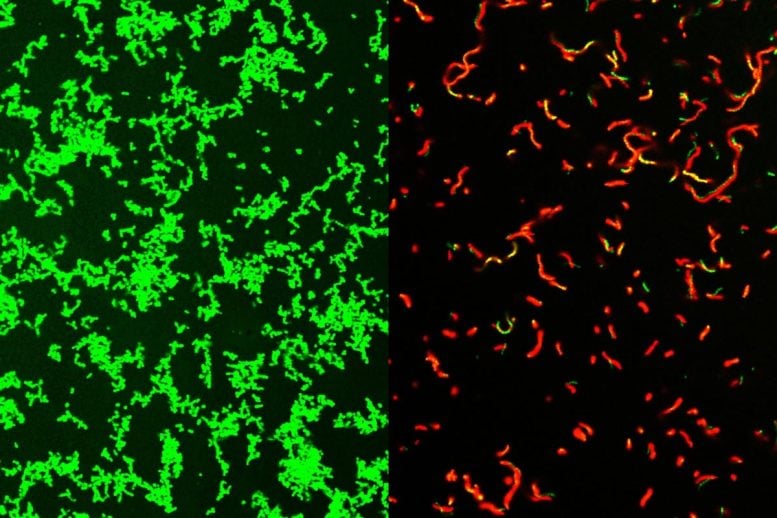
Researchers at Washington University School of Medicine in St. Louis have developed a compound that is effective against common bacteria that can lead to rare, dangerous illnesses. This image shows untreated Streptococcus pyogenes bacterial culture full of healthy microbes, labeled green (left). After treatment by GmPcide, the dish is full of dead bacteria (red; right). Credit: Zongsen Zou, Ph.D, Washington University in St Louis
Broad-Spectrum Antimicrobial Activity
“All of the gram-positive bacteria that we’ve tested have been susceptible to that compound. That includes enterococci, staphylococci, streptococci, C. difficile, which are the major pathogenic bacteria types,” said Caparon, the co-senior author. “The compounds have broad-spectrum activity against numerous bacteria.”
It’s based on a type of molecule called ring-fused 2-pyridone. Initially, Caparon and Hultgren had asked Almqvist to develop a compound that might prevent bacterial films from attaching to the surface of urethral catheters, a common cause of hospital-associated urinary tract infections. Discovering that the resulting compound had infection-fighting properties against multiple types of bacteria was a happy accident.
The team named their new family of compounds GmPcides (for gram-positive-icide). In past work, the authors showed that GmPcides can wipe out bacteria strains in petri dish experiments. In this latest study, they decided to test it on necrotizing soft-tissue infections, which are fast-spreading infections usually involving multiple types of gram-positive bacteria, for which Caparon already had a working mouse model. The best known of these, necrotizing fasciitis or “flesh-eating disease,” can quickly damage tissue severely enough to require limb amputation to control its spread. About 20% of patients with flesh-eating disease die.
Clinical Implications
This study focused on one pathogen, Streptococcus pyogenes, which is responsible for 500,000 deaths every year globally, including flesh-eating disease. Mice infected with S. pyogenes and treated with a GmPcide fared better than untreated animals in almost every metric. They had less weight loss, the ulcers characteristic of the infection were smaller, and they fought off the infection faster.
The compound appeared to reduce the virulence of the bacteria and, remarkably, speed up post-infection healing of the damaged areas of the skin.
It is not clear how GmPcides accomplish all of this, but microscopic examination revealed that the treatment appears to have a significant effect on bacterial cell membranes, which are the outer wrapping of the microbes.
“One of the jobs of a membrane is to exclude material from the outside,” Caparon said. “We know that within five to ten minutes of treatment with GmPcide, the membranes start to become permeable and allow things that normally should be excluded to enter into the bacteria, which suggests that those membranes have been damaged.”
This can disrupt the bacteria’s own functions, including those that cause damage to their host, and make the bacteria less effective at combating the host’s immune response to infections.
Challenges and the Road Ahead
In addition to their antibacterial effectiveness, GmPcides appear to be less likely to lead to drug-resistant strains. Experiments designed to create resistant bacteria found very few cells able to withstand treatment and thus pass on their advantages to the next generation of bacteria.
Caparon explained that there is a long way to go before GmPcides are likely to find their way into local pharmacies. Caparon, Hultgren and Almqvist have patented the compound used in the study and licensed it to a company, QureTech Bio, in which they have an ownership stake, with the expectation that they will be able to collaborate with a company that has the capacity to manage the pharmaceutical development and clinical trials to potentially bring GmPcides to market.
Hultgren said that the kind of collaborative science that created GmPcides is what is needed to treat intractable problems like antimicrobial resistance.
“Bacterial infections of every type are an important health problem, and they are increasingly becoming multi-drug resistant and thus harder to treat,” he said. “Interdisciplinary science facilitates the integration of different fields of study that can lead to synergistic new ideas that have the potential to help patients.”
Reference: “Dihydrothiazolo ring-fused 2-pyridone antimicrobial compounds treat Streptococcus pyogenes skin and soft tissue infection” 2 August 2024, Science Advances.
DOI: 10.1101/2024.01.02.573960







Be the first to comment on "“Happy Accident” Leads to Potent Weapon Against Flesh-Eating Superbugs"