
A lightning-inspired process creates ammonia from air. The approach may support future green energy solutions.
Researchers at the University of Sydney have used artificial lightning to create a more efficient technique for producing ammonia, a chemical essential to modern life. Ammonia plays a key role in fertilizers that contribute to nearly half of global food production.
The team developed a simplified process to produce ammonia (NH₃) directly in its gaseous form. In contrast, earlier methods developed by other labs yielded ammonium (NH₄⁺) in solution, which demands additional energy and conversion steps to obtain usable ammonia gas.
Traditionally, ammonia is produced using the Haber-Bosch process, a method with a substantial environmental impact due to its high carbon emissions. This approach also requires large-scale infrastructure and proximity to inexpensive natural gas sources to remain economically viable.
The chemical process that fed the world, and the Sydney team looking to revolutionize it
Ammonia was once so highly valued—primarily in the form of bird droppings—that it became a source of conflict.
The development of the Haber-Bosch process in the 19th century enabled the large-scale production of synthetic ammonia, transforming both agriculture and industry. Today, approximately 90 percent of the world’s ammonia is still produced using this method.
“Industry’s appetite for ammonia is only growing. For the past decade, the global scientific community, including our lab, wants to uncover a more sustainable way to produce ammonia that doesn’t rely on fossil fuels.
“Currently, generating ammonia requires centralized production and long-distance transportation of the product. We need a low-cost, decentralized and scalable ‘green ammonia’,” said lead researcher Professor PJ Cullen from the University of Sydney’s School of Chemical and Biomolecular Engineering and the Net Zero Institute. His team has been working on ‘green ammonia’ production for six years.
“In this research, we’ve successfully developed a method that allows air to be converted to ammonia in its gaseous form using electricity. A huge step towards our goals.”
The research was published in Angewandte Chemie.
Ammonia is composed of three hydrogen atoms, making it a promising carrier and source of hydrogen for energy applications. It also offers potential for storing and transporting hydrogen, as the hydrogen can be extracted by a process known as “cracking,” which separates the molecules.
Because of its chemical properties, ammonia is being explored as a carbon-free fuel alternative. This has drawn attention from the shipping sector, which contributes roughly 3 percent of global greenhouse gas emissions.
Cracking a chemical conundrum
Professor Cullen’s team’s new method to generate ammonia works by harnessing the power of plasma, by electrifying or exciting the air.
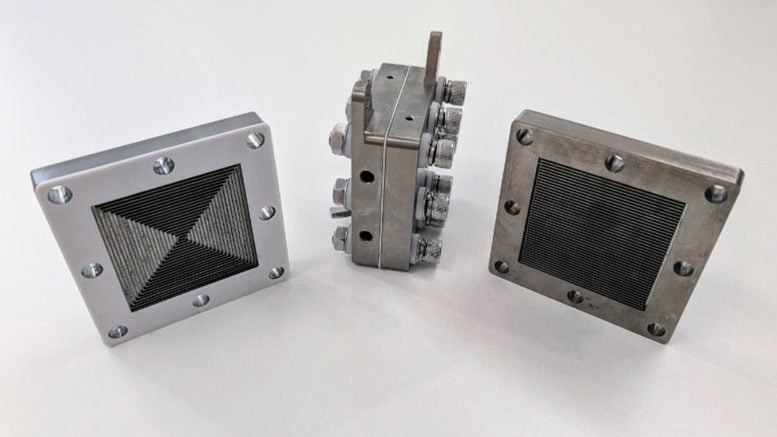
But the star is a membrane-based electrolyzer, a seemingly non-descript silver box, where the conversion to gaseous ammonia happens.
The plasma column used to kickstart the process for ‘green ammonia.’ Credit: PJ Cullen/ Plasmaleap
During the Haber-Bosch process, ammonia (NH3) is made by combining nitrogen (N2) and hydrogen (H2) gases under high temperatures and pressure in the presence of a catalyst (a substance that speeds up a chemical reaction).
The plasma-based method Professor Cullen’s team developed uses electricity to excite nitrogen and oxygen molecules in the air. The team then passes these excited molecules to the membrane-based electrolyser to convert the excited molecules to ammonia.
The researchers said this is a more straightforward pathway for ammonia production.
Professor Cullen said the findings signal a new phase in making green ammonia possible. The team is now working on making the method more energy efficient and competitive compared to the Haber-Bosch process.
“This new approach is a two-step process, namely combining plasma and electrolysis. We have already made the plasma component viable in terms of energy efficiency and scalability.
“To create a more complete solution to a sustainable ammonia productive, we need to push the energy efficiency of the electrolyser component,” Professor Cullen said.
Reference: “Regulating Multifunctional Oxygen Vacancies for Plasma-Driven Air-to-Ammonia Conversion” by Wanping Xu, Jiaqian Wang, Tianqi Zhang, Jungmi Hong, Qiang Song, Zhongkang Han and Patrick Cullen, 22 April 2025, Angewandte Chemie International Edition.
DOI: 10.1002/anie.202508240
Never miss a breakthrough: Join the SciTechDaily newsletter.
2 Comments
Excuse me, but isn’t this process about a century old?
I imagine they’re using a combination of the birkeland eyde proces but enriched with hydrogen from electrolysis to make ammonia instead of just making NO2 from air. Its old but worth revisiting.