Yale Engineers Charging Toward Better Lithium Oxygen Batteries
Yale researchers have devised a method that brings marketable Li-O2 batteries closer to reality, improving both the batteries’ performance and the ability to study them.
In recent years, lithium-oxygen batteries have intrigued researchers with their potential. They can store at least two to three times the energy as lithium-ion batteries can, which is the current standard for consumer electronics, so laptops could theoretically run longer on a single charge and electric cars would drive farther.
But they’re not quite there yet. For now, Li-O2 batteries operate sluggishly and have short lives. Compounding matters, it’s hard to get a sense of how to fix that because figuring out the exact nature of their chemistry has proved tricky.
But researchers in the lab of Prof. André D. Taylor in Chemical & Environmental Engineering have devised a method that brings marketable Li-O2 batteries closer to reality, improving both the batteries’ performance and the ability to study them. Their findings are published online in ACS Nano Letters. Won-Hee Ryu, a former post-doc in Taylor’s lab and now an assistant professor in the Department of Chemical and Biological Engineering at Sookmyung Women’s University, is the lead author.
One of the big problems with Li-O2 batteries is their production of oxides, and its effect on the electrode. “What happens is the catalysts on the electrode surface get buried with the oxide, so it’s no longer an effective catalyst,” Taylor said. Those solids build up on the electrodes, where the catalysts are placed, leading to an early battery death.
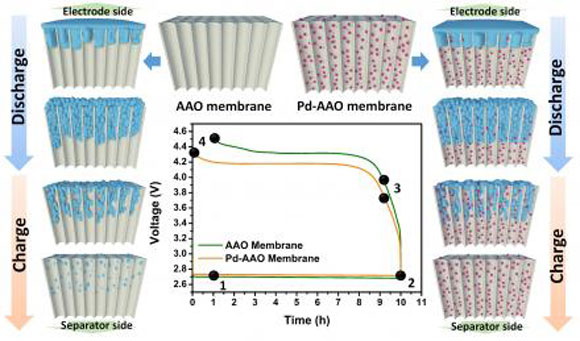
In a previous study, the researchers mitigated this effect by inserting a nonconductive porous membrane that dispersed the catalyst sites between the electrode and the separator. Doing so kept the lithium oxide formation from building up on the oxygen electrode catalysts. The catalysts on the nonconductive support facilitated oxygen evolution – a key step in recharging a battery – even when the oxide formation is far from the electrode.
But they also saw room for improvement. In the new study, the researchers replaced the membrane material, polyacrylonitrile (PAN), with anodic aluminum oxide (AAO). Palladium nanoparticles were dispersed as catalytic sites.
“The brittleness of the AAO membranes allows them to be cross-sectioned without destroying the well-defined pore structure, thus preserving the morphology of integrated lithium-oxide products,” Ryu said. “Therefore, the AAO membrane backbone offers a facile and effective way to observe cross-sectional features of the products at different electrochemical states and to investigate the growth mechanism for reaction.”
Based on that idea, the researchers demonstrated that the oxidation is possible at long distances – up to 20 micrometers – from the electrode.
“PAN is a polymer, so it could break down if you’re doing recycling,” Taylor said. “But anodic aluminum oxide is a very stable oxide and it doesn’t lead to any unexpected side reactions. It works better and gives a better perspective on what’s happening electrochemically in the process.”
The modification not only led to a more efficient battery cell function, but it allowed the researchers to examine the composition of discharge products and the catalytic sites using Raman and X-ray photoelectron spectroscopy.
For future studies, Taylor said they will try different catalysts with the AAO membrane and explore new Li-O2 architectures.
Reference: “A New Design Strategy for Observing Lithium Oxide Growth-Evolution Interactions Using Geometric Catalyst Positioning” by Won-Hee Ryu, Forrest S. Gittleson, Jinyang Li, Xiao Tong and André D. Taylor, 21 June 2016, Nano Letters.
DOI: 10.1021/acs.nanolett.6b00856








Be the first to comment on "A New Design Strategy for Better Lithium Oxygen Batteries"