
Artistic interpretation of experiment, done at high pressure (applied through diamond anvils) and high temperature, shedding light on the location of Earth’s supply of argon (particles zooming toward the core). The findings answer the question of whether argon atoms, chemically inert at Earth’s surface, can react with nickel in the core to form a thermodynamically stable compound. Credit: Adam Connell/Lawrence Livermore National Laboratory
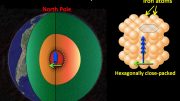
The radioactive decay of potassium-40 in Earth’s core is considered one of the main sources of heat powering the geodynamo that generates Earth’s magnetic field. However, only about half the expected amount of argon that should be produced by potassium-40 decay is found in Earth’s mantle, crust, and atmosphere. The “missing” argon may be present in Earth’s core, but a more definitive conclusion is hindered by a lack of information about the reactivity of argon (a chemically inert noble gas at ambient conditions) with nickel and iron (the core’s main constituents) at the temperatures and pressures in Earth’s core.
At Advanced Light Source (ALS) Beamline 12.2.2, researchers performed x-ray diffraction experiments on a nickel–argon mixture while subjecting it to pressures greater than 1.5 million times Earth’s atmospheric (surface) pressure and temperatures above 2000 K (1,700 C or 3,100F). The results strongly indicated that a new compound does indeed form—an alloy of 50% argon and 50% nickel. Theoretical calculations revealed that argon engages in significant electron transfer with nickel, resulting in a material with the crystal structure of an intermetallic alloy, with the argon and nickel equally interspersed in a solid solution.
The results open up the possibility of argon being bound to nickel in the Earth’s core and may help solve outstanding geological questions about the location of argon (the “missing argon paradox”). Moreover, this study highlights that extreme pressures and temperatures lead to new chemistry rules and underscores the need for close synergy between experimental and theoretical studies in this new frontier of chemical properties under extreme thermodynamic conditions.
Reference: “A High-Pressure Compound of Argon and Nickel: Noble Gas in the Earth’s Core?” by Adebayo A. Adeleke, Martin Kunz, Eran Greenberg, Vitali B. Prakapenka, Yansun Yao and Elissaios Stavrou, 1 October 2019, ACS Earth and Space Chemistry.
DOI: 10.1021/acsearthspacechem.9b00212







Be the first to comment on "Argon: Not So Noble in Earth’s Core – Solving the Missing Argon Paradox"