Researchers at Hokkaido University have created molecules capable of rotating internally when exposed to light, potentially revolutionizing molecular switches, computing, and drug development. Inspired by natural processes, this breakthrough offers new avenues for precise molecular manipulation and applications in targeted therapies and bioactive systems.
Molecules that are induced by light to rotate bulky groups around central bonds when exposed to light could lead to the creation of light-activated bioactive systems, molecular switches, among other applications.
Researchers at Hokkaido University, led by Assistant Professor Akira Katsuyama and Professor Satoshi Ichikawa at the Faculty of Pharmaceutical Sciences, have extended the toolkit of synthetic chemistry by making a new category of molecules that can be induced to undergo an internal rotation on interaction with light.
Similar processes are believed to be important in some natural biological systems. Synthetic versions might be exploited to perform photochemical switching functions in molecular computing and sensing technologies, or in bioactive molecules including drugs. They report their findings in Nature Chemistry.
Advancements in Photochemistry
“Achieving a system like ours has been a significant challenge in photochemistry,” says Katsuyama. “The work makes an important contribution to an emerging field in molecular manipulation.”
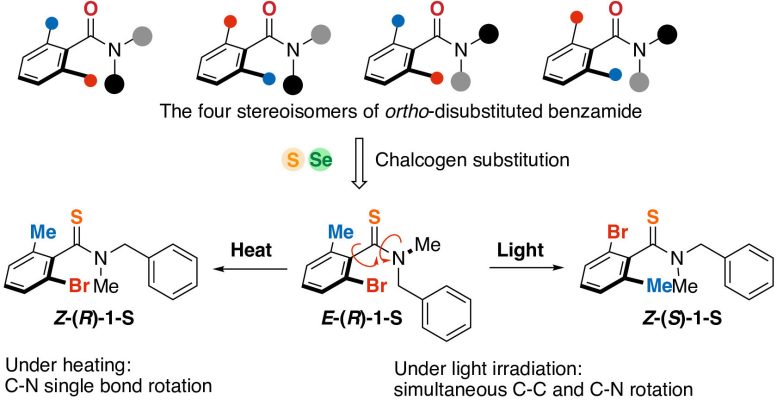
In this study, sulfur or selenium atoms were introduced into a benzamide derivative (top). The resulting molecules underwent isomerization when exposed to light or heat. Credit: Shotaro Nagami, et al. Nature Chemistry. February 28, 2024

The molecules synthesized in this study form different isomers when irradiated with blue light. Credit: Akira Katsuyama
Insights into the possibilities for light to significantly alter molecular conformations have come from examining some natural proteins. These include the rhodopsin molecules in the retina of the eye, which play a crucial role in converting light into the electrical signals that create our sense of vision in the brain. Details are emerging of how the absorption of light energy can induce a twisting rearrangement of part of the rhodopsin molecule, required for it to perform its biological function.
“Mimicking this in synthetic systems might create molecular-level switches with a variety of potential applications,” Katsuyama explains.
Innovations in Molecular Rotation
A key innovation by the Hokkaido team was to achieve photo-induced (i.e., light-driven) rotation of molecular groups around a series of chemical bonds that incorporate a nitrogen atom together with other bonded carbon atoms.
The rotational properties were enabled by adding molecular components that contained an atom from the ‘chalcogen’ group of elements in the periodic table, specifically sulfur or selenium, to a simple organic molecule: an amide compound. This brought a new level of control and versatility to synthetic photo-induced rotational systems.

In this study, sulfur or selenium atoms were introduced into a benzamide derivative (top). The resulting molecules underwent isomerization when exposed to light or heat. Credit: Shotaro Nagami, et al. Nature Chemistry. February 28, 2024
Some of the chemical groups that rotate around the central bonds were relatively large, based on rings of six bonded carbon atoms. This facilitated the large-scale molecular changes that might be required for practical use in molecular switching systems.
In addition to demonstrating the photo-induced changes, the team also performed theoretical calculations that gave insights into the likely mechanisms by which the rearrangements proceeded. The team also explored the effects of temperature on the transformations. The combination of theoretical and experimental work should help guide future research toward exploring and controlling modifications to the systems already achieved.
“Our next research priority is focused on the potential of our methods for making new bioactive molecules activated by light. These could be applied in biological research or possibly developed as drugs,” Ichikawa concludes.
Using light to activate the conformational changes allows control over where and when the changes occur. This could be vital for precisely targeted applications in biological systems, including eventual therapeutic possibilities.
Reference: “Photoinduced dual bond rotation of a nitrogen-containing system realized by chalcogen substitution” by Shotaro Nagami, Rintaro Kaguchi, Taichi Akahane, Yu Harabuchi, Tohru Taniguchi, Kenji Monde, Satoshi Maeda, Satoshi Ichikawa and Akira Katsuyama, 28 February 2024, Nature Chemistry.
DOI: 10.1038/s41557-024-01461-9
The study was funded by the Japan Society for the Promotion of Science, the Japan Agency for Medical Research and Development, the Japan Science and Technology Agency, and the Akiyama Life Science Foundation.






Be the first to comment on "Mimicking Nature: New Molecular Switches Transform Synthetic Chemistry"